Amisulpride tablet and method for preparing same
A technology of amisulpride tablets and tablets, which is applied in the direction of pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of improving taste, rapid onset of effect, poor solubility of amisulpride, Problems such as poor fluidity and formability can achieve the effect of improving drug compliance, improving dissolution rate and reducing possibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
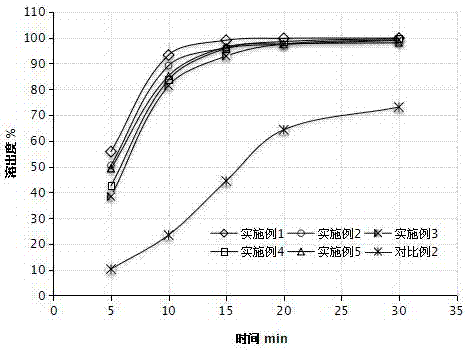
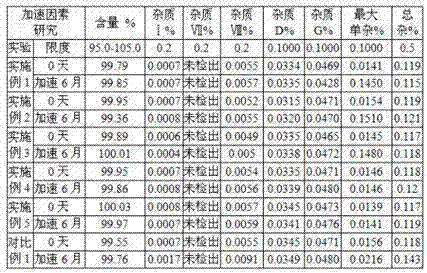
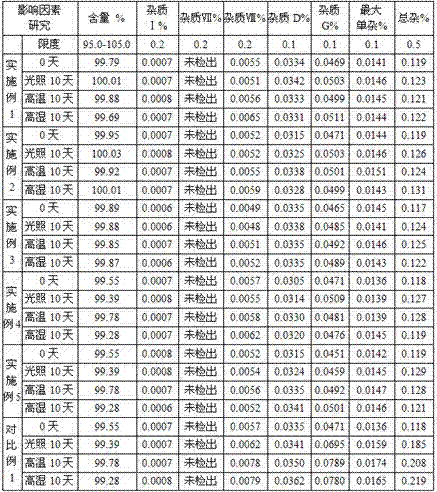
Examples
Embodiment 1
[0041] Components of film-coated tablets (mass unit: mg), each tablet contains 200 mg of amisulpride:
[0042] Amisulpride 200.0g
[0043] Lactose 53.6g
[0044] Microcrystalline Cellulose 107.2g
[0045] Sodium carboxymethyl starch 4.0g
[0046] Pregelatinized starch 32.0g
[0047] Magnesium Stearate 3.2g
[0048] Film coating premix:
[0049] Opadry Gastric Film Coating Powder 48.5g
[0050] Aspartame 0.25g
[0052] Peach Flavor Essence 0.25g
[0053] Film-coated tablet preparation process:
[0054] (1) Weigh the raw materials in proportion, pass the amisulpride, filler, disintegrant and binder through a 60-mesh sieve and mix evenly;
[0055] (2) Add an appropriate amount of wetting agent water 200g to the mixture mixed in the previous step, pass through a 20-mesh sieve to granulate, dry the prepared granules in an oven at 50-60°C, pass through a 20-mesh sieve for granulation;
Embodiment 2
[0061] Composition of amisulpride film-coated tablet (mass unit: mg), each tablet contains 200mg of amisulpride:
[0062] Amisulpride 200.0g
[0063] Lactose 200.0g
[0064] Sodium carboxymethyl starch 46.0g
[0065] Povidone k30 50.0g
[0066] Magnesium Stearate 4.0g
[0067] Film coating premix:
[0068] Opadry Gastric Film Coating Powder 49.0g
[0069] Acesulfame K 0.10g
[0070] Sodium chloride 0.75g
[0071] Cherry Flavor 0.15g
[0072] Film-coated tablet preparation process:
[0073] (1) Weigh the raw materials in proportion, pass the amisulpride, filler, disintegrant and binder through a 60-mesh sieve and mix evenly;
[0074] (2) Add an appropriate amount of wetting agent water 200g to the mixture mixed in the previous step, pass through a 20-mesh sieve to granulate, dry the prepared granules in an oven at 50-60°C, pass through a 20-mesh sieve for granulation;
[0075] (3) Add lubricant talcum powder to the granules prepared in the previous step, and mix well; ...
Embodiment 3
[0080] Composition of amisulpride film-coated tablet (mass unit: mg), each tablet contains 100mg of amisulpride:
[0081] Amisulpride 100.0g
[0082] Lactose 75.0g
[0083] Microcrystalline Cellulose 200.0g
[0084] Mannitol 75.0g
[0085] Croscarmellose Sodium 12.0g
[0086] Pregelatinized starch 35.0g
[0088] Film coating premix:
[0089] Opadry Gastric Film Coating Powder 49.5g
[0090] Aspartame 0.15g
[0091] Sodium chloride 0.30g
[0092] Strawberry Peach Flavor 0.05g
[0093] Film-coated tablet preparation process:
[0094] (1) Weigh the raw materials in proportion, pass the amisulpride, filler, disintegrant and binder through a 60-mesh sieve and mix evenly;
[0095] (2) Add an appropriate amount of wetting agent water 200g to the mixture mixed in the previous step, pass through a 20-mesh sieve to granulate, dry the prepared granules in an oven at 50-60°C, pass through a 20-mesh sieve for granulation;
[0096] (3) Add the lubricant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com