Azo monomer, and azo polymer prepared by polymerization of the azo monomer
An azo polymer, azo technology, applied in the field of metal ion sensors and metal ion traps, can solve the problems of characteristics and applications without research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
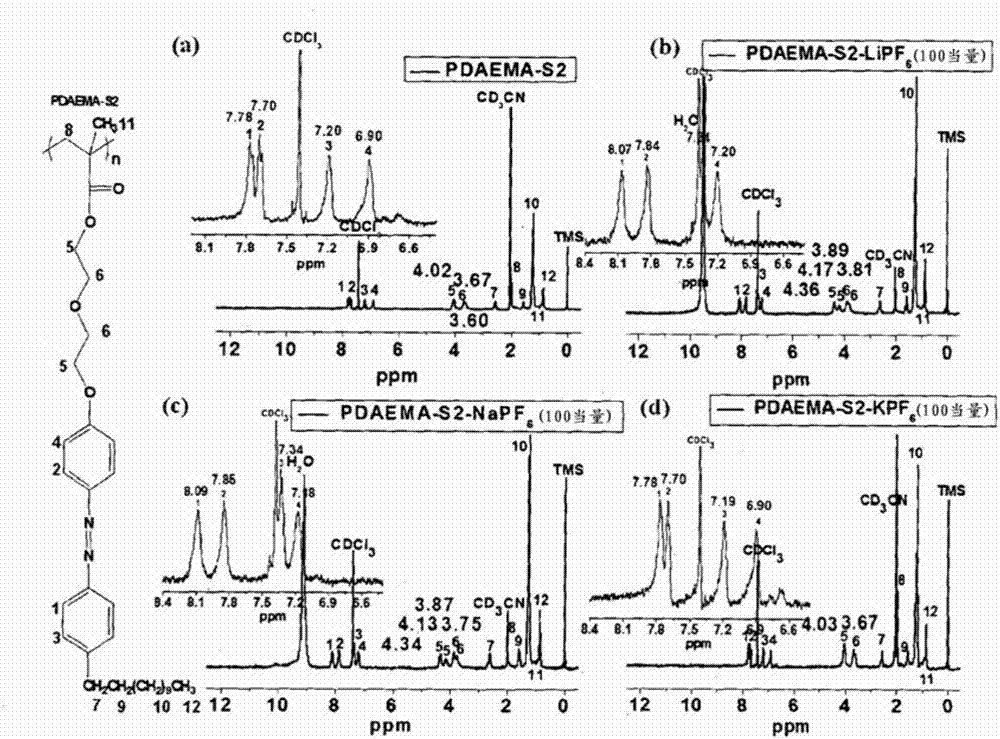
[0068] The length of such an alkyleneoxy group can be easily controlled using appropriate precursors as in Preparation Examples 2 to 4 described below. refer to figure 1 , the glass transition temperature, nematic liquid crystal transition temperature and melting point of azo polymers tend to decrease as the length of the alkyleneoxy group increases. Therefore, by controlling the length of the alkyleneoxy group of Chemical Formula 1, the heat resistance and the like of the azo polymer can be easily controlled.
[0069] In Chemical Formula 1, m may be an integer of 1 to 5, and when m is 2 or more, two or more R 2 can all be the same, or two or more R 2 At least one of can be different. More suitably, m may be an integer from 1 to 3 or the integer 1. Referring to the following experimental examples, the shorter the length of the alkyleneoxy group, the stronger the bonding force between the azo polymer and the specific metal ion may become. Meanwhile, regardless of the lengt...
preparation example 1
[0095] Preparation Example 1: Synthesis of 4-dodecyl-4'-hydroxyazobenzene (DHAB)
[0096] p-Dodecylaniline (97%, 5.00 g, 18.60 mmol), HCl (4.85 mL, 55.00 mmol), NaNO 2 (1.32 g, 18.60 mmol), 156 mL of THF and 39 mL of distilled water were placed in a 500 mL 3-neck round bottom flask, and the solution was stirred at 0 °C for 2 hours under nitrogen atmosphere. Subsequently, phenol (1.77 g, 18.60 mmol), K 2 CO 3 (1.30g, 19.10mmol), Na 2 CO 3 (1.44 g, 27.10 mmol), 128 mL THF and 32 mL H 2 0 and the solution was stirred at room temperature for an additional 12 hours. Thereafter, the solvent was removed from the reaction solution using a rotary evaporator. The solid thus obtained was put into a separatory funnel containing 300 mL of dichloromethane (MC) and 300 mL of distilled water and vigorously shaken, and then the aqueous solution layer in which the unreacted salt was dissolved was removed. 10.0 g of anhydrous magnesium sulfate was introduced into the remaining MC soluti...
preparation example 2
[0100] Preparation Example 2: Synthesis of 2-(4-dodecylazobenzene-4'-oxy)ethanol (DAEA-S1)
[0101] DHAB (5.00 g, 13.60 mmol) synthesized in Preparation Example 1, K 2 CO 3 (1.14 g, 8.25 mmol) and 50 mL of diglyme were placed in a 2-neck round bottom flask, and the solution was stirred at room temperature under nitrogen atmosphere for 30 minutes. 2-Chloroethanol (2.20 g, 27.28 mmol) was introduced into the solution, and the solution was stirred at 140°C for an additional 48 hours. Pour the reaction solution into 1LH 2 Precipitate in O and stir for 1 h to dissolve unreacted K 2 CO 3 , and then filtered to obtain a solid precipitate containing unreacted DHAB and product. The precipitate was dissolved in 200 mL of chloroform, 10.0 g of anhydrous magnesium sulfate was introduced thereinto, and the solution was stirred for 30 minutes and filtered to remove traces of water dissolved in the precipitate. The solvent of the filtered chloroform solution was evaporated, and then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com