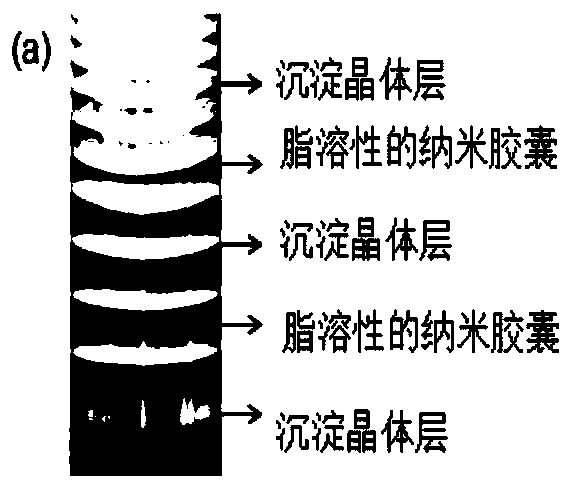
A self-assembled multi-pulse release method for fat-soluble drugs
A fat-soluble drug, multi-pulse technology, applied in pharmaceutical formulations, organic active ingredients, medical preparations with non-active ingredients, etc. Increase fixing performance, wide application range and obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of a self-assembled multi-pulse release device for fat-soluble drugs loaded with cholesterol nanocapsules (precipitation ring layer is calcium hydrogen phosphate crystals)
[0036] (1) Weigh 0.017g of cholesterol and dissolve it in 10ml of chloroform, then weigh 0.017g of surfactant sodium dodecylbenzene sulfonate and dissolve it in 50ml of twice distilled water. Add the chloroform solution into 50ml of the surfactant-containing solution, and obtain a transparent nanocapsule solution with a concentration of about 0.01mol / L by evaporation to remove the solvent. Such as image 3 As shown, the diameter of the nanocapsules carrying fat-soluble cholesterol is between 60-70nm as measured by DLS, which proves that the fat-soluble cholesterol is encapsulated by the surfactant to form stable nanocapsules.
[0037] (2) After mixing the above-mentioned nanocapsule solution, calcium chloride solution with a concentration of 0.1mol / L and gelatin solution with ...
Embodiment 2
[0040] Example 2 Preparation of a self-assembled multi-pulse release device for fat-soluble drugs loaded with prednisone nanocapsules in sodium cholate (precipitation ring layer is calcium hydrogen phosphate crystals)
[0041] Weigh 0.017g prednisone fat-soluble drug and dissolve it in 10ml chloroform, then weigh 0.17g surfactant sodium cholate and dissolve it in 50ml double distilled water. The chloroform solution of prednisone is added into 50 ml of sodium cholate solution, and the nanocapsule solution with a concentration of about 0.01 mol / L is prepared by evaporation to remove the solvent.
[0042] Remaining steps are identical with embodiment 1.
Embodiment 3
[0043] Example 3 Preparation of a self-assembled multi-pulse release device for fat-soluble drugs loaded with paclitaxel nanocapsules (precipitation ring layer is calcium hydrogen phosphate crystals)
[0044] (1) Weigh 0.017g of paclitaxel fat-soluble drug and dissolve it in 10ml of chloroform, then weigh 0.17g of surfactant sodium dodecyl sulfonate and dissolve it in 50ml of double distilled water. Add the chloroform solution into 50 ml of sodium dodecylsulfonate solution, and prepare a nanocapsule solution with a concentration of about 0.01 mol / L by evaporation to remove the solvent.
[0045] Remaining steps are identical with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com