A kind of preparation method of idebenone
A technology of idebenone and benzoquinone, which is applied in the field of preparation of idebenone, can solve the problems of long reaction route, low yield and high cost, and achieves the advantages of few reaction steps, easy-to-obtain raw materials and low-cost raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
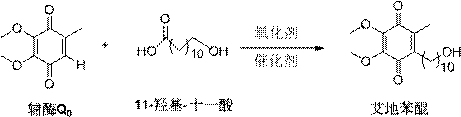
[0033] A typical implementation of the present invention provides a preparation method of idebenone, comprising the steps of:
[0034] 1) Coenzyme Q 0 Put 11-hydroxyundecanoic acid in a ratio of 1:3 into a container with a thermometer, add copper chloride, copper sulfate, copper acetate, silver carbonate, silver oxide, palladium chloride or a metal salt of palladium acetate As a catalyst, the catalyst is added in an amount of coenzyme Q 0 0.1~2eq of molar weight;
[0035] 2) Add ethanol and water for ultrasonic dissolution, and raise the temperature to 40-90°C;
[0036] 3) Under the protection of oxygen, add oxidizing agent 30% hydrogen peroxide;
[0037] 4) Continue the heat preservation reaction for 1-4 hours, add water to quench the reaction, and the reaction system is extracted
[0038] Washing with water, drying, distillation under reduced pressure, and column chromatography to obtain crystal 6-(10-hydroxydecyl)-2,3-dimethoxy-5-methyl-1,4-benzoquinone, namely idebenon...
Embodiment 1
[0054] coenzyme Q 0 preparation of
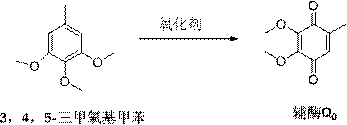
[0055] Weigh 3.6g of raw material 3,4,5-trimethoxytoluene, add 0.33ml of concentrated sulfuric acid to 10ml of ethanol solution, stir and dissolve, slowly add 4ml of oxidant peroxyacetic acid with a concentration of 10mol / L dropwise, drop within 10 minutes Complete reaction at 35°C for 2 hours, TLC point plate monitoring raw materials have been completely reacted, stop the reaction, add 20ml of water to quench the reaction, extract with dichloromethane, organic layer with 5% FeCl 3 30 mL was washed 3 times, and washed with water until the pH of the aqueous layer reached 6. Combined organic layers were dried and treated, recrystallized with petroleum ether to obtain coenzyme Q in red needle-like crystals 0 , yield 86%, melting point 55-58 ℃.
[0056] Preparation of idebenone
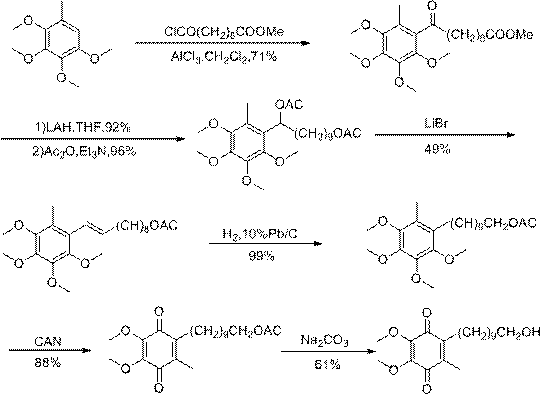
[0057] Weigh Coenzyme Q 0 1.2g, 1.50g of 11-hydroxyundecanoic acid, 0.2g of copper chloride catalyst, put in a 250ml three-necked round bottom flask with a th...
Embodiment 2
[0068] coenzyme Q 0 preparation of
[0069] Weigh 3.6g of the raw material 3,4,5-trimethoxytoluene, add 0.75ml of pure formic acid to 10ml of methanol solution, stir and dissolve, slowly add 10ml of oxidant cerium ammonium nitrate with a concentration of 2mol / L, and drop it within 10 minutes Complete, react at 30°C for 1.5 hours, TLC spot plate monitors that all the raw materials have reacted, stop the reaction, add 20ml of water to quench the reaction, extract with dichloromethane, and use 5% FeCl for the organic layer 3 30 mL was washed three times, and washed with water until the pH of the aqueous layer reached 5.5. Combined organic layers were dried and treated, recrystallized with petroleum ether to obtain coenzyme Q in red needle-like crystals 0 , yield 78%, melting point 55-58 ℃.
[0070] Preparation of idebenone
[0071] Weigh Coenzyme Q 0 1.2g, 1.50g of 11-hydroxyundecanoic acid, 0.1g of copper sulfate catalyst, put in a 250ml three-necked round-bottomed flas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com