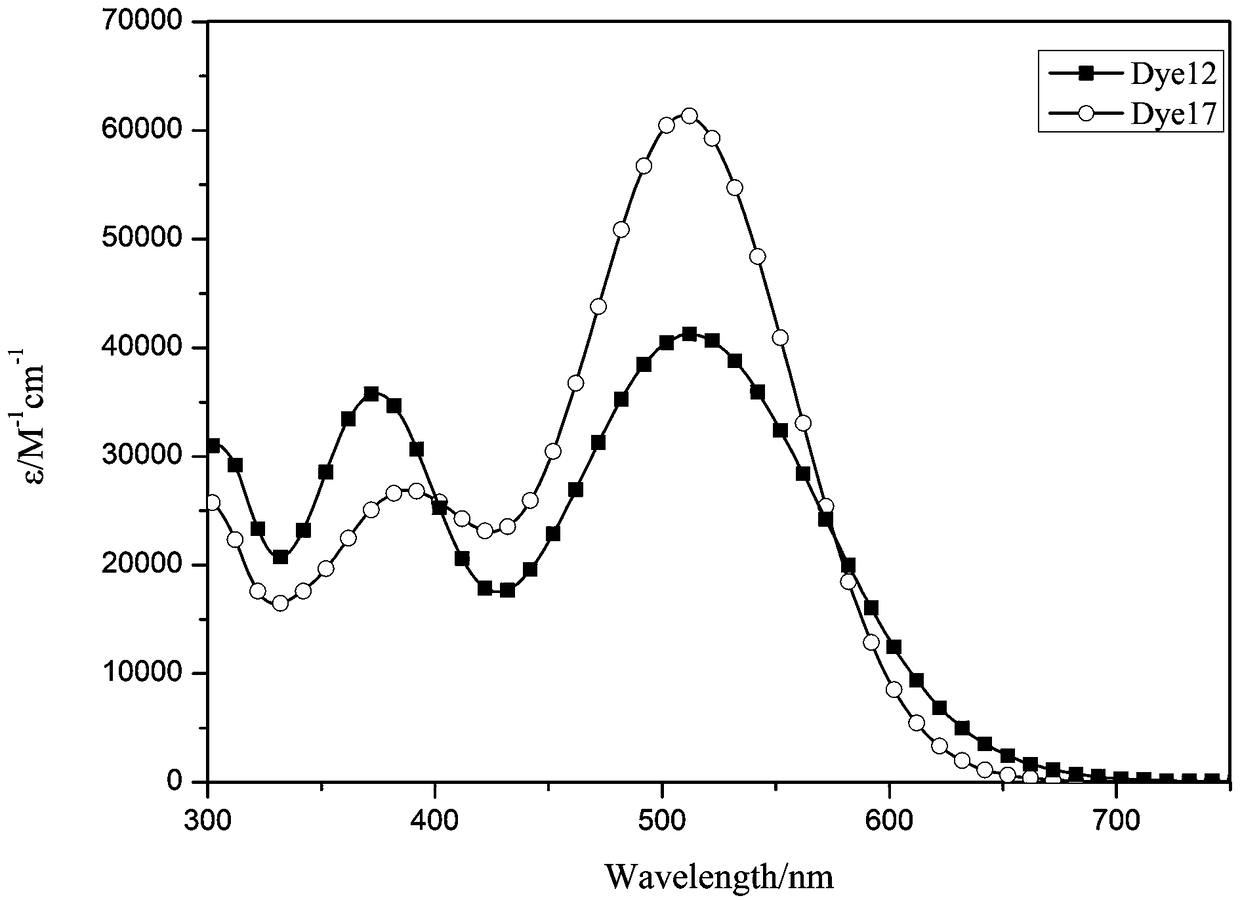
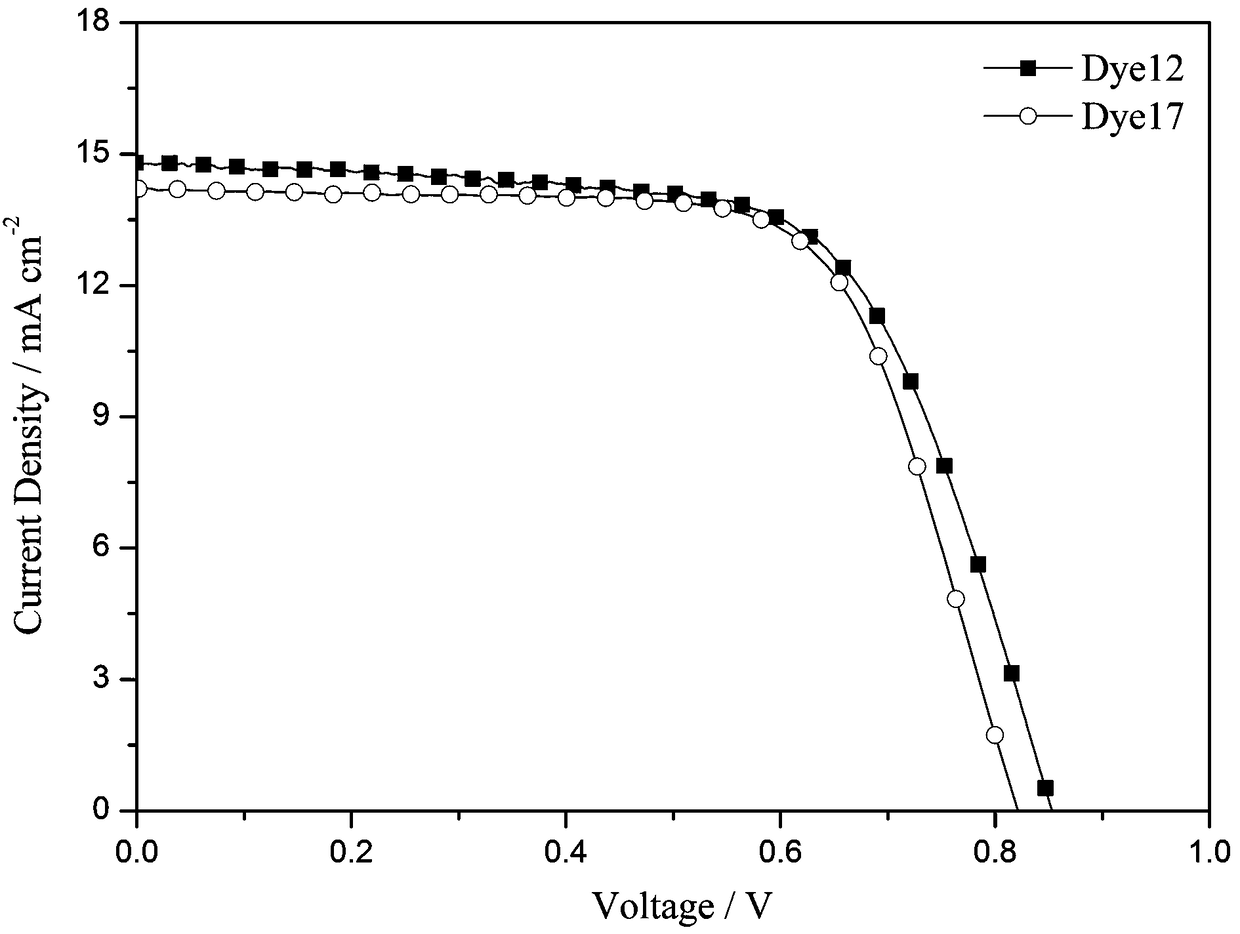
Organic dyes containing trithienopyrrole-thiophene and their applications in dye-sensitized solar cells
An organic dye, thiophene pyrrole technology, applied in organic dyes, organic chemistry, azo dyes, etc., can solve the problem of narrow spectral absorption range, and achieve the effect of improving photovoltaic performance, improving efficiency, and optimizing dye energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the preparation of organic dye 16
[0043] The synthetic route is as follows:
[0044]
[0045] The raw material 1 used in this example was prepared according to the literature Q.Yu, W.Fu, J.Wan, X.Wu, M.Shi, H.Chen.ACSAppl.Mater.Interfaces2014, 6, 5798-5809; raw material 10, 4-hexyloxyaniline and 3-bromo-2-thiophene zinc bromide According to literature Z.Wang, M.Liang, Y.Tan, L.Ouyang, Z.Sun, S.Xue.J.Mater.Chem.A ,2015,3,4865–4874 prepared from 4-hexyl-5-bromo-2-thiophene carboxaldehyde according to literature H.Lee, H.Jo, D.Kim, S.Biswas, G.D.Sharma, J.Ko.Dyes Pigments, 2016, 129, 209-219. Synthesis, other reagents can be obtained commercially.
[0046] Synthesis of Intermediate 2:
[0047] Into a 100mL single-necked round-bottom flask, add 3.75g of raw material 1 and 20mL of N-N dimethyldimethylformamide, then add 2.13g of N-bromosuccinimide to the system in batches, and keep the Light reaction for 8 hours; quenched with water, extracted with eth...
Embodiment 2
[0066] Embodiment 2: the synthesis of organic dye 17
[0067] The synthetic route is as follows:
[0068]
[0069] The raw material 7 and the intermediate 10 can be synthesized with reference to the preparation method of Example 1, and other reagents can be purchased commercially.
[0070] Synthesis of intermediate 13:
[0071] Under the protection of argon, in a 100mL three-necked round-bottomed flask, 0.784g of intermediate 12, 0.296g of 3-hexyl-2-bromothiophene, 0.011g of tetrakis(triphenylphosphine)palladium and 15mL of toluene were added successively, and the reaction system was heated After reacting at 110°C for 12 hours, cool to room temperature, concentrate by rotary evaporation, and purify the crude product by column chromatography (eluent: petroleum ether / dichloromethane=25 / 1~10 / 1) to obtain 0.476g of intermediate 13, pale yellow Viscous liquid, 72% yield. 1 H NMR (400MHz, CDCl 3 ): δ7.52(d, J=8.8Hz, 2H), 7.15(d, J=5.2Hz, 1H), 7.08-7.06(m, 3H), 6.92(d, J=5.4Hz...
Embodiment 3
[0081] Application of the dye 12 prepared in Example 1 in the preparation of dye-sensitized solar cells. Its specific use method is the same as the use method disclosed in the invention patent application with the application number 201510015966.7 and the invention title "A Carbazole-type Photosensitizing Dyestuff and Its Preparation Method and Application". Test light source: AM 1.5 (solar simulator-Oriel 91160-1000, 300W), data acquisition using Keithley 2400 digital source meter. see test results image 3 , the open circuit voltage of the battery (V oc ) is 852mV, short-circuit current density (J sc ) is 14.8mA cm -2 , the fill factor (FF) is 0.66, and the photoelectric conversion efficiency is 8.37%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com