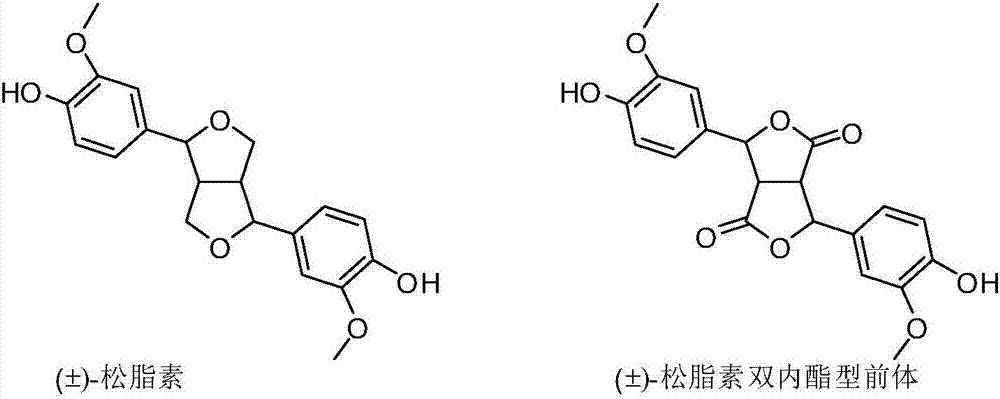
A pinoresinol precursor and a synthetic method thereof
A technology of pinoresin and precursors, applied in the field of organic chemical synthesis, to achieve the effects of easy acquisition, reduced reaction cost, and simple and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
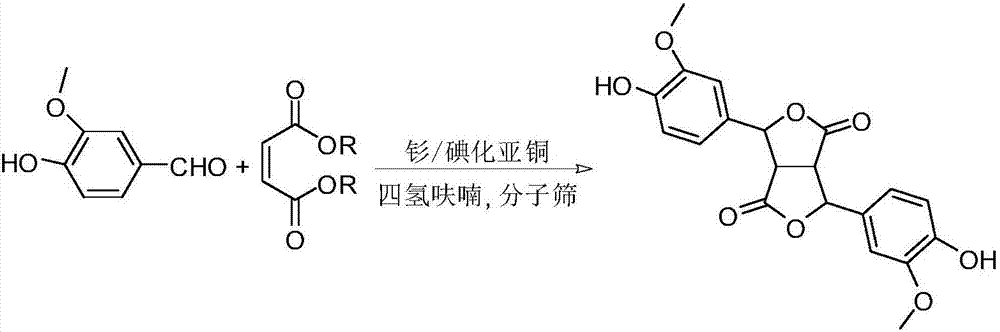
[0016] Add 3 g of freshly prepared samarium metal powder, 3.9 g of cuprous iodide, and 6.8 g of potassium iodide in turn into the reaction vessel, then add 100 mL of tetrahydrofuran that has been dehydrated and dried in advance, and stir magnetically. Add 3 g of vanillin, 5 mL of dimethyl maleate, and 5 g of 4A molecular sieves. The color of the reaction solution became dark within 2h, and the reaction was continued for 8h. Add 50mL dilute hydrochloric acid (2mol L -1 ) to terminate the reaction, the reaction mixture was post-treated to obtain a crude product, and then further purified through a chromatographic column to obtain a dilactone-type pinoresin precursor with a yield of 80%.
[0017] White solid, melting point>250℃. 1 HNMR (500MHz, CDCl 3 )δppm 9.85(s,2H),7.09-6.94(m,2H),6.87-6.75(m,4H),6.03-6.02(m,1H),5.97(m,1H),3.96-3.93(m,6H ),3.77-3.74(m,2H); 13 C NMR (125MHz, CDCl 3 )δppm 177.6, 173.2, 139.4, 137.6, 137.1, 136.5, 133.1, 132.2, 130.0, 126.8, 125.9, 124.0, 1...
example 2
[0019] According to the method of Example 1, using 3A molecular sieve instead of 4A molecular sieve, a dilactone-type pinoresin precursor was obtained with a yield of 73%.
example 3
[0021] According to the method of Example 1, diethyl maleate was used instead of dimethyl maleate to obtain a dilactone-type pinoresin precursor with a yield of 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com