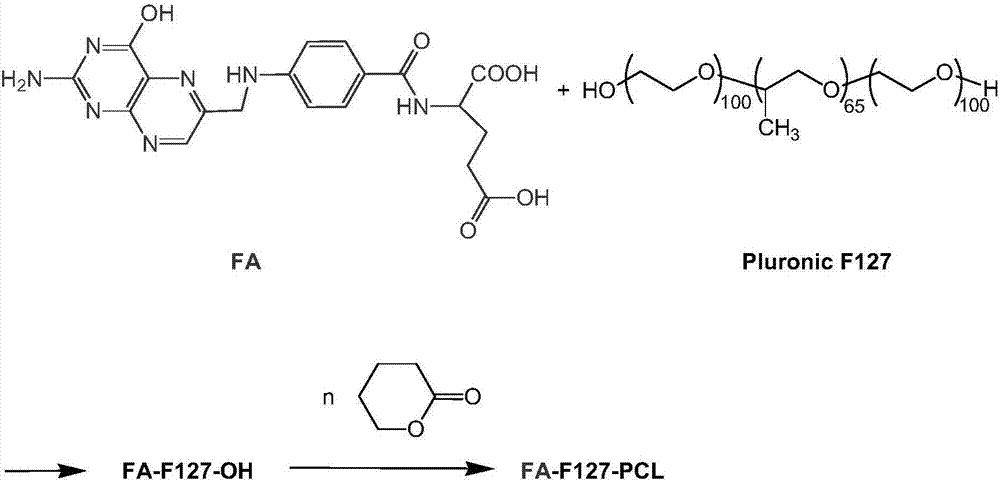
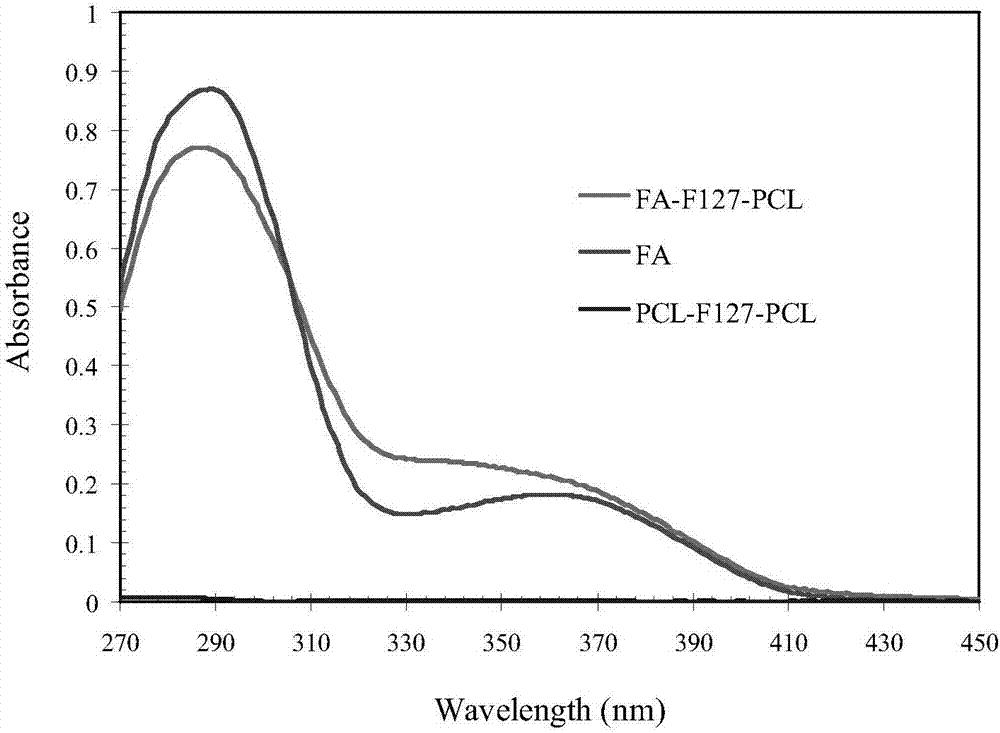
FA-F127-PCL folate-targeted copolymer as well as preparation method and application thereof
A copolymer and polymer technology, applied in the field of biomedicine, can solve the problems of limiting the effectiveness of the treatment of cancer and other diseases, and achieve the effect of good drug release effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation method of FA-F127-PCL copolymer of the present invention is as follows:
[0031] (1) Take 0.71g (1.61mmol) of FA and 0.36g (1.76mmol) of 1,3-dicyclohexylcarbodiimide (DCC), mix them into 70ml of anhydrous DMSO in stirring, stir at room temperature for 48 Hour. Then, 20 g of dried F127 and 0.20 mg (1.60 mmol) of 4-dimethylaminopyridine (DMAP) were added thereto, stirred at 25° C. for 48 hours, and then centrifuged at 5000 r / min for 5 min. The supernatant was dialyzed with 3500 molecular weight cut-off dialysis bag for 3 hours in normal DMSO environment, and then dialyzed with distilled water for 24 hours. Spin dry with CH 2 Cl 2 After dissolving, sink into frozen ether to purify twice, take the precipitate and dry it to obtain FA-F127-OH; the weight of the obtained copolymer is 18.0 g, and the yield is 81.7%.
[0032] (2) Use FA-F127-OH as a macromolecular initiator and stannous octoate as a catalyst to initiate ring-opening polymerization of the cycl...
Embodiment 2
[0035] Embodiment 2 FA-F127-PCL copolymer of the present invention embeds paclitaxel
[0036] 1. Preparation of FA-F127-PCL nanoparticles embedded with paclitaxel
[0037] Weigh 15 mg of FA-F127-PCL polymer and 5 mg of paclitaxel (Paclitaxel, PTX) into a stoppered test tube, add 3 ml of tetrahydrofuran (THF) to dissolve. The THF solution was then dispersed in 15 g of ultrapure water, poured into a dialysis bag and dialyzed for 24 hours to remove unencapsulated paclitaxel.
[0038] 2. Determination of paclitaxel embedding rate and drug loading
[0039] Get 0.3ml of the nanoparticle aqueous solution in step 1 to freeze-dry, then add 0.7ml of acetonitrile-water (7: 3v / v) mixed solution to dissolve, then use high performance liquid chromatography (HPLC) to test, the test conditions are as follows: C18 column, Take 1.0ml·min -1 The flow rate of acetonitrile-water (7:3v / v) was used as the mobile phase, the peak area was detected at 227nm, and the paclitaxel concentration in the s...
Embodiment 3
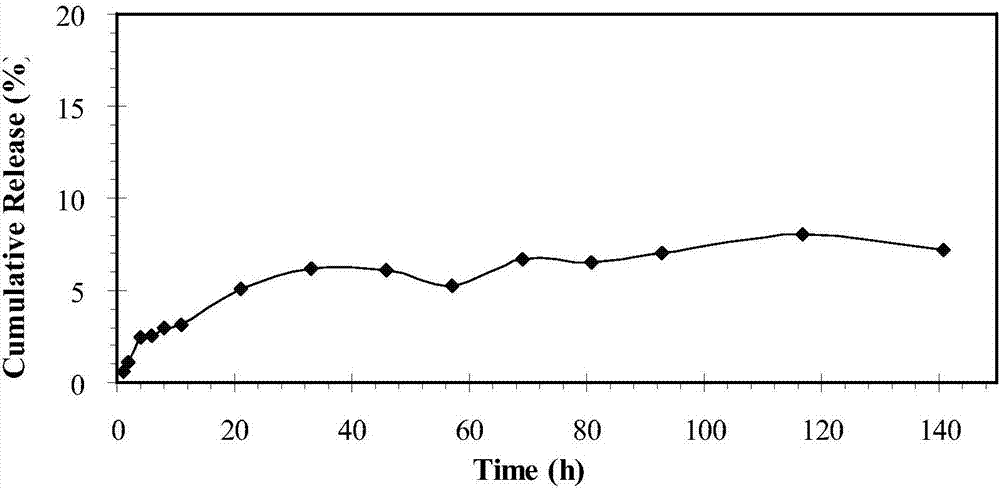
[0044] 1. In Vitro Release of Paclitaxel-Encapsulated FA-F127-PCL Nanoparticles
[0045] Take 10ml of dialysate and place it in the corresponding dialysis bag; put the dialysis bag (molecular cut-off 3.5kDa) in a jar containing 100ml of PBS solution. The jar is placed in a constant temperature shaker, and the solution in the bottle is kept shaking at 37.5°C. At the set time point, take 5 mL from the jar to release the external liquid, and then add 5 mL of PBS buffer solution to keep the total amount at 100 mL. The paclitaxel content in the release liquid was determined by HPLC, and the test conditions were the same as above. It can be seen from the test results (see image 3 ), the paclitaxel embedded in the nano-particles presented a better slow-release process; after 141 hours of release, only about 8% of the paclitaxel was released.
[0046] 2. In vitro release of paclitaxel-embedded FA-F127-PLA nanoparticles
[0047] Weigh 3mg FA-F127-PLA (Mn 27000, PLA 51.7wt%, FA 12....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com