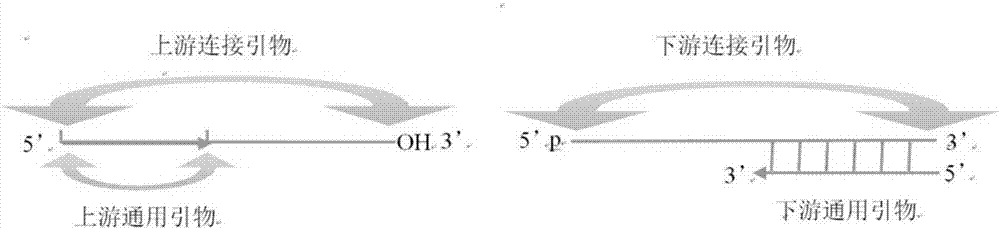
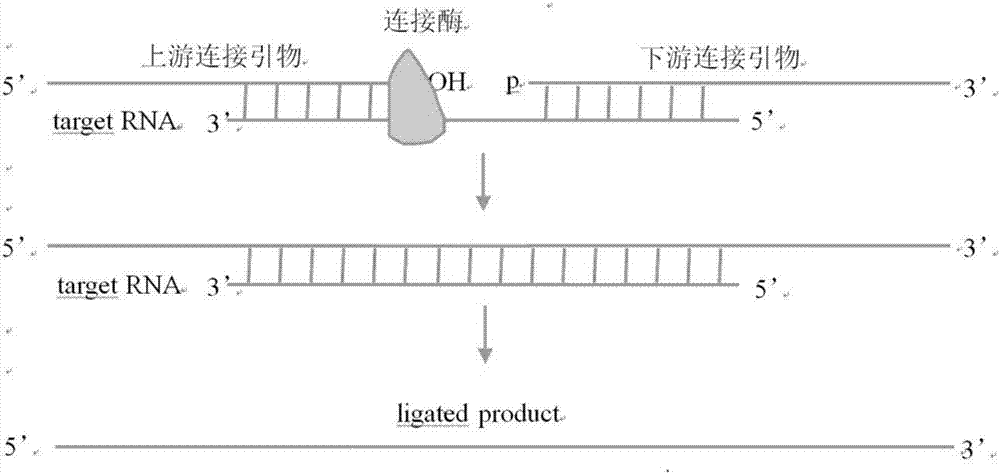
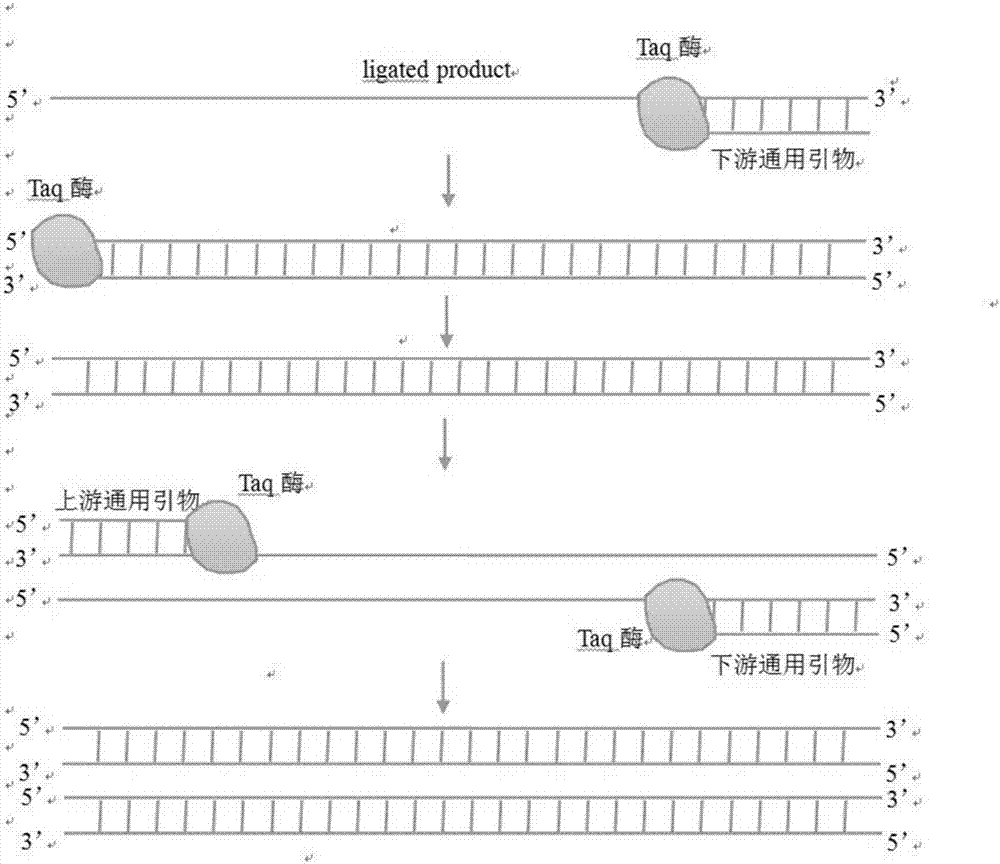
Method and kit for detecting influenza A virus series by ligase
An influenza A virus and ligase technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve the problems of long time, low detection sensitivity, low throughput, etc., and achieve rapid detection and detection throughput. High, improve the effect of diagnosis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the composition of kit of the present invention and the manufacturer and specification of main raw material
[0041] Composition Specification quantity main ingredient RNA extraction solution 1300ul / tube 1 tube Tris, EDTA Connect the reaction tube 10ul / tube 24 tubes Primers and ligases for upstream and downstream connection of influenza A virus H1, H3, H5, H7 subtypes Fluorescence PCR reaction tube 30ul / tube 24 tubes Hot start Taq enzyme, UDG enzyme, universal primers, specific fluorescent probes for each subtype of influenza A virus (H1, H3, H5, H7) negative control 100ul / tube 1 tube Sterilized purified water positive control 100ul / tube 1 tube Inactivated Influenza A Virus H1 Subtype Sample
[0042]Wherein, the connection primer includes the above-mentioned sequence of SEQ ID NO: 1-8, the universal primer includes the above-mentioned sequence of SEQ ID NO: 9-10, and the fluorescent p...
Embodiment 2
[0049] Embodiment 2: the using method of kit of the present invention
[0050] 1. Sample processing and RNA extraction
[0051] 1) Shake the throat swab preservation tube for 10-30 seconds (to make the cells fully fall into the liquid), and take 0.5ml-1.0ml of the exfoliated cell sample (if the number of cells is small, the volume can be increased to 2.0ml)
[0052] 2) Centrifuge the numbered samples at 10,000rpm for 3 minutes, discard the supernatant;
[0053] 3) Add 1ml sterilized saline to the sample pellet, wash the pellet, centrifuge at 10,000rpm for 3 minutes, and discard the supernatant;
[0054] 4) Add 50µl RNA extraction solution to the sample pellet, shake for 60 seconds, fully suspend the cell pellet, and set aside;
[0055] Extraction of RNA can also be performed using other known methods.
[0056] 5) Quality control treatment
[0057] Take the centrifuge tubes containing the positive quality control and negative quality control, centrifuge at 10,000rpm for 30 ...
Embodiment 3
[0076] Embodiment 3: The basis for setting the reference value of the kit of the present invention and the detection situation of clinical samples
[0077] Use this kit and the control kit to test the clinical samples, and use the re-check kit for detection if the two are inconsistent, and divide the samples into a positive group and a negative group according to the control and re-check test results, and compare the Ct values detected by this kit , so as to determine the reference value of this kit. When the Ct value of this kit is greater than 37, it belongs to the negative group. When the Ct value of this kit is less than or equal to 37, it belongs to the positive group. Therefore, the Ct value greater than 37 is used as the reference value (CUTOFF value) of this kit.
[0078] The schematic diagram of the detection results using the detection kit of the present invention is as follows: Figure 4~6 shown. It can be clearly seen from the figure that the detection kit of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com