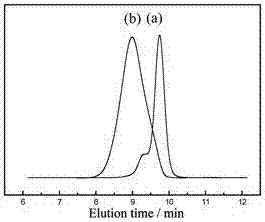
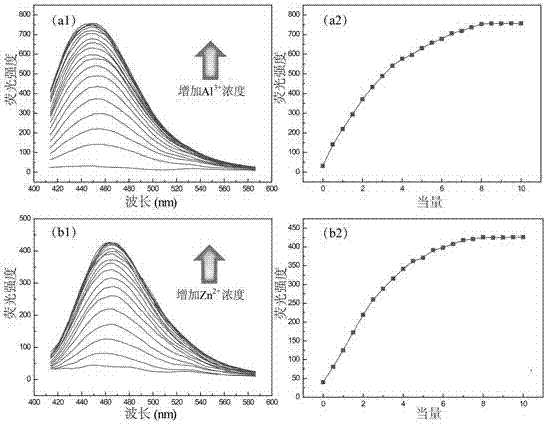
Amphiphilic temperature-sensitive block copolymer-based sensor, as well as preparation method and application thereof
A block copolymer and amphiphilic technology, applied in the field of chemical fluorescent materials, can solve the problems of poor water solubility, low sensitivity reliability and weak biocompatibility of metal ion sensors, achieve excellent water solubility and improve detection sensitivity , the effect of fluorescence intensity enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: Preparation of salicylaldehyde hydrazone SH
[0059]
[0060] References (G. L. Backes, D. M. Neumann, B. S. Jursic, Synthesis and antifungal activity of substituted salicylaldehyde hydrazones, hydrazides and sulfohydrazides, Bioorg. Med. Chem. 22(17) (2014) 4629-4636.).
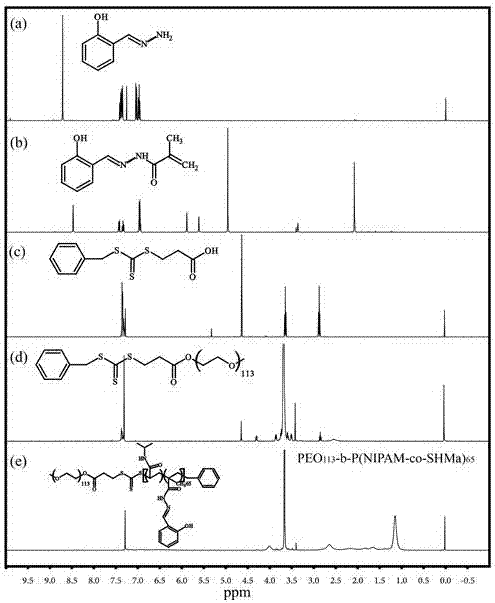
[0061] (1.18 g, 20.00 mmol) hydrazine hydrate was dissolved in ethanol (20.00 ml), and a mixed solution of salicylaldehyde (2.45 g, 20.00 mmol) and ethanol (10.00 ml) was added dropwise, and the reaction temperature was raised to 70.0 ℃, react for 30 min. After the reaction, the solvent was removed to obtain a crude product, which was washed three times with cold diethyl ether (20.00 ml) to obtain a white scaly solid product (2.04 g, yield: 75 %). 1 H NMR (CDCl 3 , δ, ppm; Fig. S1 (a)): 8.71 (1H, ArCH=N-), 7.31-7.44 (1H, -C(CH 3 )=CHH), 6.92-7.07 (4H, ArH).
Embodiment 2
[0062] Example 2: Preparation of fluorescent probe SHMA based on salicylaldehyde hydrazone
[0063]
[0064] Dissolve salicylaldehyde hydrazone (1.50 g, 11.00 mmol) in dry CH 2 Cl 2 (20.00 ml), methacryloyl chloride (3.45 g, 33.00 mmol) was added dropwise in an ice-water bath. Reflux at 25.0 °C for 3 h. The organic layer was dried with anhydrous sodium sulfate, and filtered, and the filtrate was rotary evaporated to obtain a crude product, and finally the crude product was passed through a silica gel column with dichloromethane as the developing solvent to obtain a gray-green solid powder. 1 H NMR (CD 3 OD, δ, ppm; Fig. 2): 8.48 (1H, ArCH=N-), 6.96-7.44(4H, ArH), 5.85 (1H, -C(CH 3 )=CHH), 5.58 (1H, -C(CH 3 )=CHH), 2.04 (3H, CH 3 C-).
Embodiment 3
[0065] Embodiment 3: the preparation of RAFT reagent BTPA
[0066]
[0067] Refer to literature (J. Skey, R. K. O'Reilly, ChemInform Abstract: Facile One-PotSynthesis of a Range of Reversible Addition-Fragmentation Chain Transfer (RAFT) Agents, Chem Commun. 40(4) (2009) 4183-4185.) :
[0068] 3-Mercaptopropionic acid (MPA) (2.50 ml, 28.65 mmol) was added dropwise to KOH (1.84 mol / L, 31.25 ml) aqueous solution, and then CS 2 (3.65ml, 28.65mmol). After stirring at room temperature for 5 h, benzyl bromide (4.95 g, 28.65 mmol) was added dropwise and heated to 80.0 °C for 12 h. After the reaction, the reaction solution was cooled to room temperature, extracted with chloroform (90.00 ml), then acidified by adding excess hydrochloric acid, and the organic phase was washed with distilled water repeatedly, and the solvent was evaporated to dryness to obtain a yellow solid (3.20 g, yield: 40.9%). 1 H NMR (CDCl 3 , δ, ppm, TMS; Fig. 2): 7.33 (5H,ArH), 4.64 (2H, ArCH 2 -), 3.65 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com