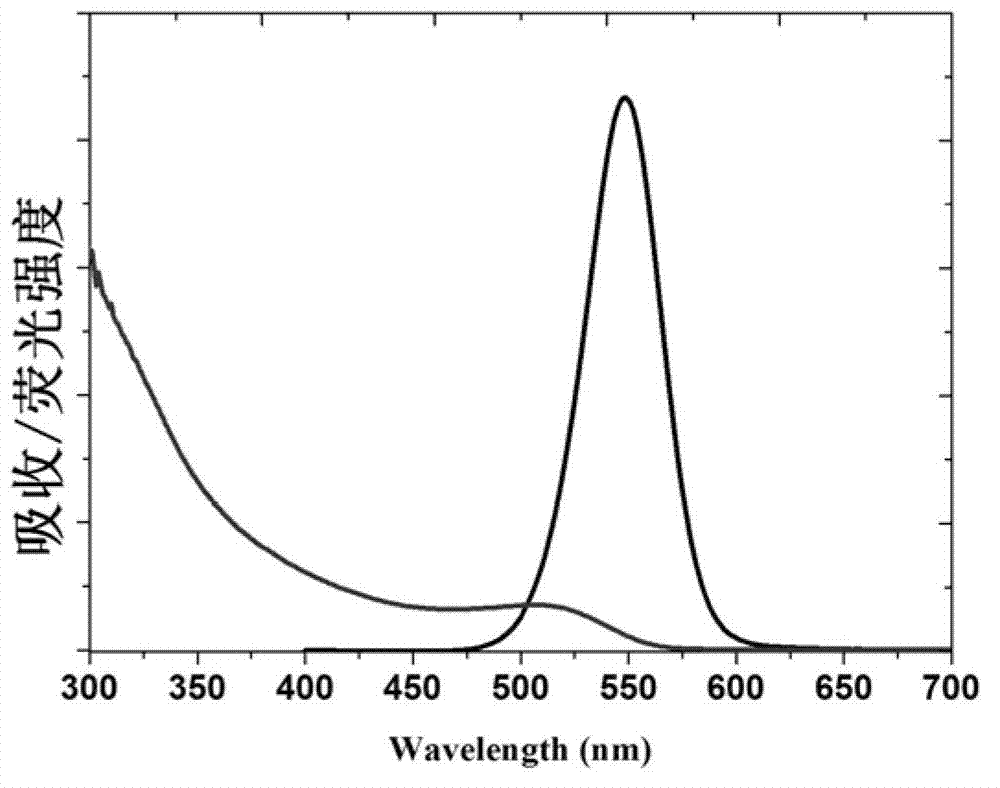
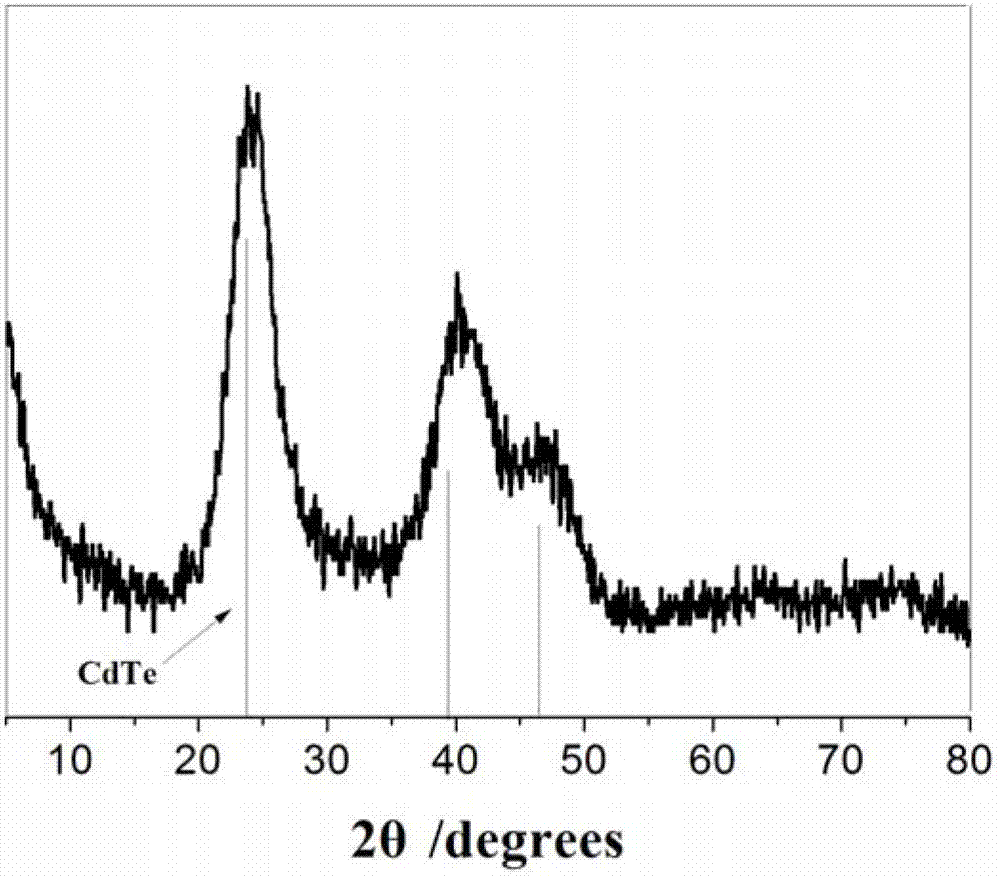
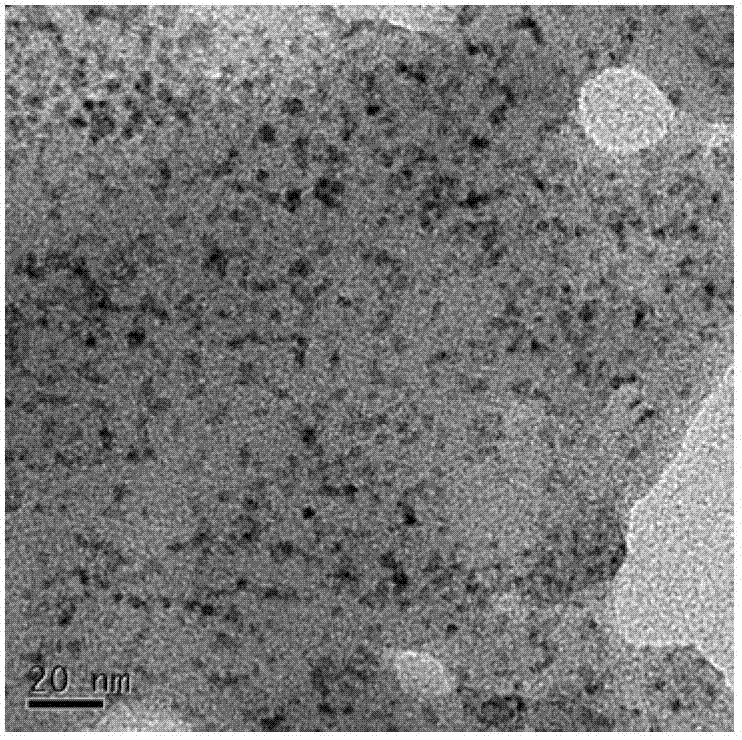
CdX quantum dot and preparation method thereof
A technology of quantum dots and cadmium salts, which is applied in the field of nanomaterial preparation, can solve the problems of low yield and harsh production conditions of CdX, and achieves the effects of high quantum yield, low preparation cost and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] First, 0.0272 g (0.1 mmol) of cadmium acetate was dissolved in deionized water to prepare 50 ml of cadmium acetate solution, and then 10 microliters (0.1 mmol) of thioglycolic acid was added dropwise. Sodium oxide solution adjusted the pH of the solution to 10, stirred for 5 minutes to obtain a cadmium source solution; dissolved 0.0052 g (0.02 mmol) of potassium tellurite in ionized water to prepare 50 ml of potassium tellurite solution, and added the cadmium source The solution was stirred for 5 minutes; then, 0.04 g (1 mmol) of sodium borohydride was added to the above solution, and stirred for 5 minutes; finally, the temperature was raised to 100 degrees Celsius and refluxed for 15 minutes. Add an equal volume of acetone to the reaction solution, centrifuge at a speed of 5000 rpm, and wash with acetone three times to obtain pure solid quantum dots with a quantum yield of 79%.
Embodiment 2
[0034] First, 0.0272 g (0.1 mmol) of cadmium acetate was dissolved in deionized water to prepare 50 ml of cadmium acetate solution, and then 10 microliters (0.1 mmol) of thioglycolic acid was added dropwise. Sodium oxide solution adjusted the pH of the solution to 10.5, stirred for 5 minutes to obtain a cadmium source solution; dissolved 0.0052 g (0.02 mmol) of potassium tellurite in ionized water to prepare 50 ml of potassium tellurite solution, and added the cadmium source The solution was stirred for 5 minutes; then, 0.08 g (2 mmol) of sodium borohydride was added to the above solution, and stirred for 5 minutes; finally, the temperature was raised to 100 degrees Celsius and refluxed for 1 hour. Add an equal volume of acetone to the reaction solution, centrifuge at a speed of 5000 rpm, and wash with acetone for 3 times to obtain pure solid quantum dots with a quantum yield of 83%.
Embodiment 3
[0036] First, 0.0272 g (0.1 mmol) of cadmium acetate was dissolved in deionized water to prepare 50 ml of cadmium acetate solution, and then 10 microliters (0.1 mmol) of thioglycolic acid was added dropwise. Sodium oxide solution adjusted the pH of the solution to 11, stirred for 5 minutes to obtain a cadmium source solution; dissolved 0.0052 g (0.02 mmol) of potassium tellurite in ionized water to prepare 50 ml of potassium tellurite solution, and added cadmium The source solution was stirred for 5 minutes; then, 0.02 g (0.5 mmol) of sodium borohydride was added to the above solution, and stirred for 5 minutes; finally, the temperature was raised to 100 degrees Celsius and refluxed for 13 hours. Add an equal volume of acetone to the reaction solution, centrifuge at a speed of 5000 rpm, and wash with acetone for 3 times to obtain pure solid quantum dots with a quantum yield of 60%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| full width at half maximum | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com