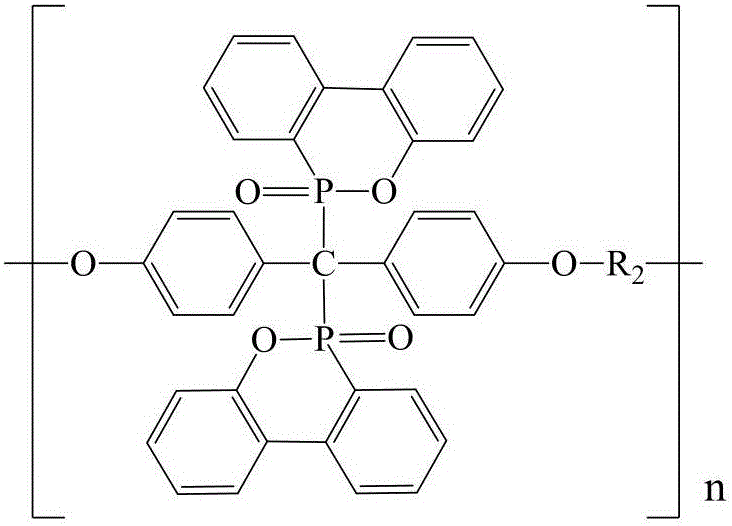
Preparation method for polyphosphoester fire retardant with dual-DOPO as branch chain
A technology of polyphosphate ester and flame retardant, which is applied in the field of preparation of polyphosphate ester flame retardant, can solve the problems such as steric hindrance, inability to obtain polyphosphoric acid flame retardant with high degree of polymerization, poor matrix compatibility, and high volatility. To achieve the effect of easy control of reaction conditions, good compatibility, and difficult migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
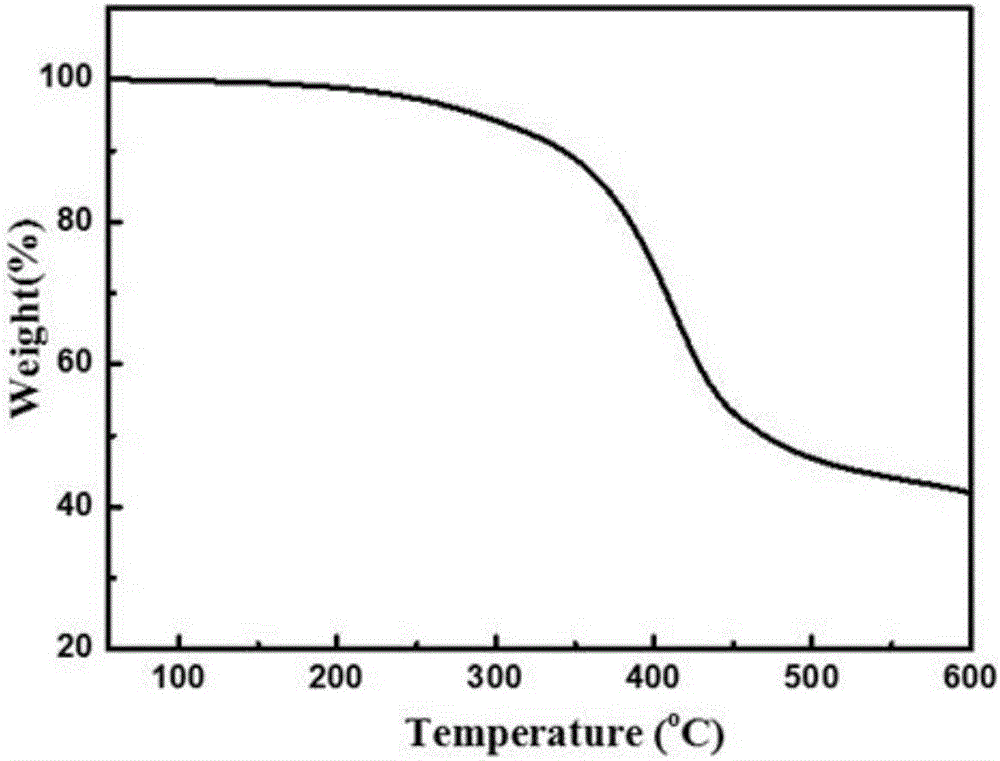
Examples
Embodiment 1
[0039] (1) Synthesis of polymeric intermediate P-PDCP
[0040] Measure 21.40g (0.10mol) of 4,4'-dihydroxybenzophenone, 19.50g (0.10mol) of phenylphosphoryl dichloride and 300mL of anhydrous acetonitrile, and add them to the In the 500mL reaction vessel with protective gas inlet and outlet, under the condition of feeding nitrogen, pre-react at 70°C for 2h, then raise the temperature to 80°C, and react until there is no HCl gas (reaction ends). The obtained precipitate was suction filtered while it was hot, washed several times with anhydrous acetonitrile, and dried in a vacuum oven at 80°C for 2 hours to obtain 29.8 g of a light yellow solid product with a yield of 88.6%.
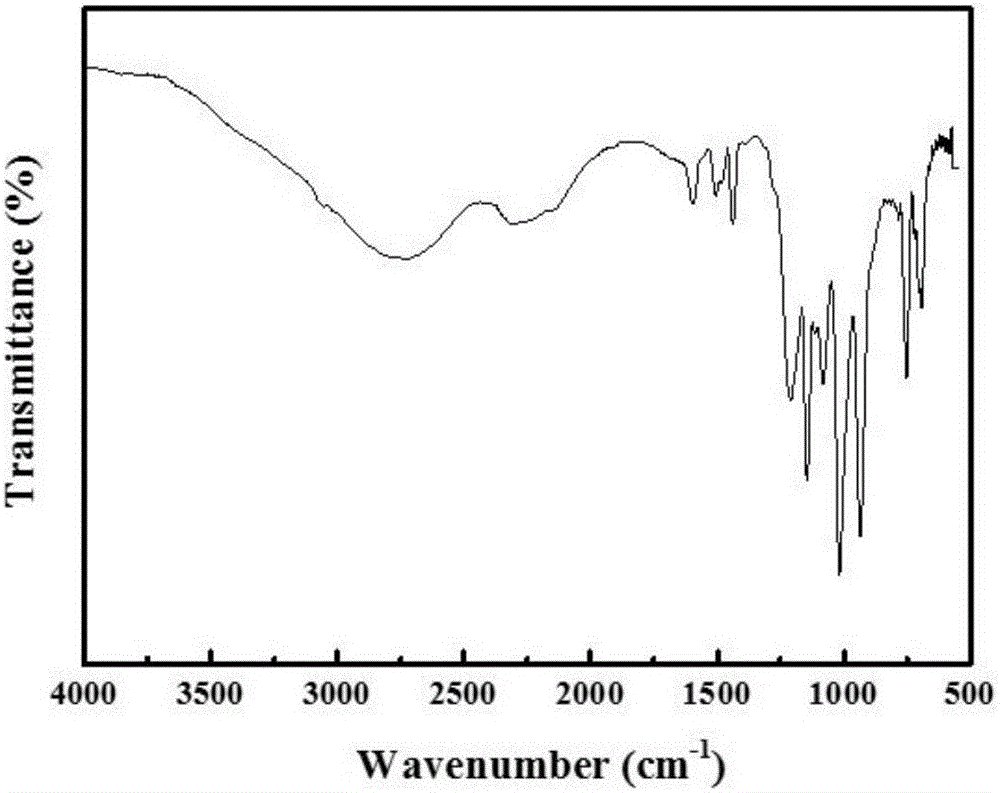
[0041] The polymeric intermediate prepared above was subjected to infrared testing. The machine model used was a Bruker Tensor 27 Fourier transform infrared spectrometer. Potassium bromide was pressed into tablets for sample preparation, and the scanning range was 4000-500cm -1 . Analyze the infrared spect...
Embodiment 2
[0052] (1) Synthesis of polymeric intermediate P-PPDC
[0053] The synthesis steps of the polymeric intermediate P-PPDC are carried out according to the synthesis steps of P-PDCP in Example 1, except that 0.10 mol of phenylphosphoryl dichloride (PDCP) in Example 1 is replaced by 0.10 mol of dichloride Phenyl phosphate (PPDC). After purification and drying, a yellow solid product was obtained with a yield of 90.6%.
[0054] According to the infrared test method in Example 1, the intermediate P-PPDC is tested, and the infrared spectrum obtained by the test is analyzed. In the infrared spectrum of the intermediate P-PPDC, O-H in 4,4'-dihydroxybenzophenone is in The strong and broad characteristic peaks at 3250-3350cm-1 and the sharp characteristic peaks of the P-Cl bond at 541-583cm-1 in phenyl phosphate dichloride (PPDC) disappear, corresponding to 929cm-1 and 1189cm-1 The characteristic peak of P-O-Ph appeared. It can be known that 4,4'-dihydroxybenzophenone and phenyl phosp...
Embodiment 3
[0060] (1) Synthesis of polymeric intermediate P-SPDPC
[0061] The synthesis steps of polymeric intermediate P-SPDPC are carried out according to the synthesis steps of P-PDCP in Example 1, except that 0.10mol of phenylphosphoryl dichloride (PDCP) in Example 1 is replaced by 0.10mol of pentaerythritol diphosphate Ester diphosphoryl chloride (SPDPC). After purification and drying, a yellow solid product was obtained with a yield of 78.9%.
[0062] According to the infrared test method in Example 1, the intermediate P-SPDPC is tested, and the infrared spectrum obtained by the test is analyzed. In the infrared spectrum of the intermediate P-SPDPC, O-H in 4,4'-dihydroxybenzophenone is in The strong and broad characteristic peak at 3250-3350cm-1 and the sharp characteristic peak of the P-Cl bond in pentaerythritol diphosphate diphosphoryl chloride (SPDPC) at 541-583cm-1 disappeared, and the positions at 929cm-1 and 1189cm-1 The corresponding characteristic peaks of P-O-Ph appear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com