Efficient CH4-SCR denitration catalyst under oxygen-enriched condition and preparation method and application thereof
A CH4-SCR, denitrification catalyst technology, applied in the field of high-efficiency CH4-SCR denitration catalyst and its preparation, can solve the problems of high requirements for equipment and pipelines, high price, reduced catalytic activity, etc., and achieve a wide activity temperature window, good Anti-SO2 performance, good selective catalytic activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Catalyst preparation.
[0048] The preparation method of the catalyst in this paper adopts the liquid phase ion exchange method,
[0049] With molecular sieve H-Beta as carrier, H-ZSM-5, H-SAPO-34 and H-MOR as control samples, Si / Al=25, In(NO 3 ) 3 4H 2 O is a precursor.
[0050] During the preparation of the catalyst, first prepare 100ml of indium nitrate solution with a concentration of 0.033M, then add 3g of molecular sieve powder to the solution, mix well and place it on a magnetic stirrer, and magnetically stir it in a constant temperature water bath at 85°C for 8h for ionization. Exchange, put the stirred solution on the Buchner funnel, filter it with a positive air pump, wash it with water until the pH of the supernatant is 7, pour off the filtrate, take out the filter cake on the filter paper and put it in the oven, and put it in the oven. Set the temperature to 80°C, then bake for 12 hours. The dried catalyst was taken out and ground, and then put into a t...
Embodiment 2
[0052] Catalyst activity evaluation.
[0053] The catalyst activity evaluation method is the temperature programmed surface reaction (TPSR) method. The concentration of each component in the reaction gas is: NO is 500ppm, CH 4 500ppm, O 2 5%, Ar is the balance gas. The total gas flow rate is 100ml / min, and the catalyst consumption is 100mg. Before the temperature program, let the reaction gas pass through the catalyst at room temperature, and absorb the reaction gas for about 1 hour until the NO x The readings of the analyzer basically did not change, indicating that the catalyst was in a state of adsorption saturation, and then the temperature program was started, from 40°C to 600°C at a rate of 4°C / min. The reaction product was detected by gas chromatography GC2014 and NO x Analyzer determination.
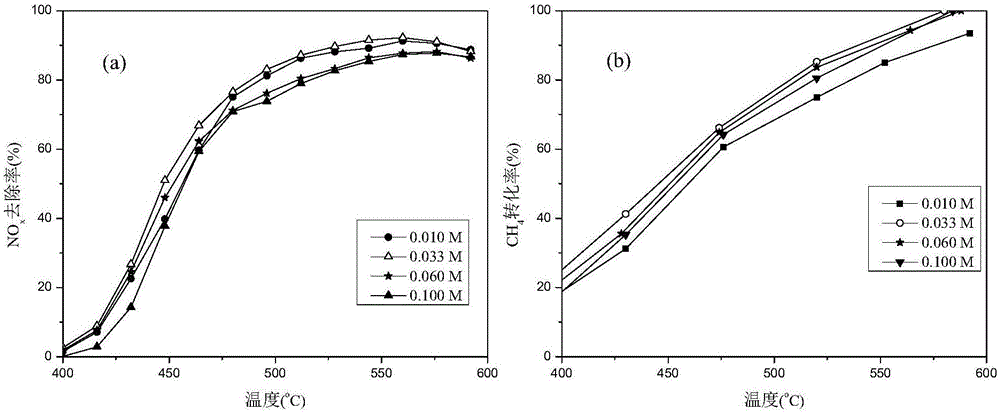
[0054] The performance analysis of the catalyst obtained under the optimal preparation conditions has been carried out under various reaction conditions, and the reaction c...
Embodiment 3
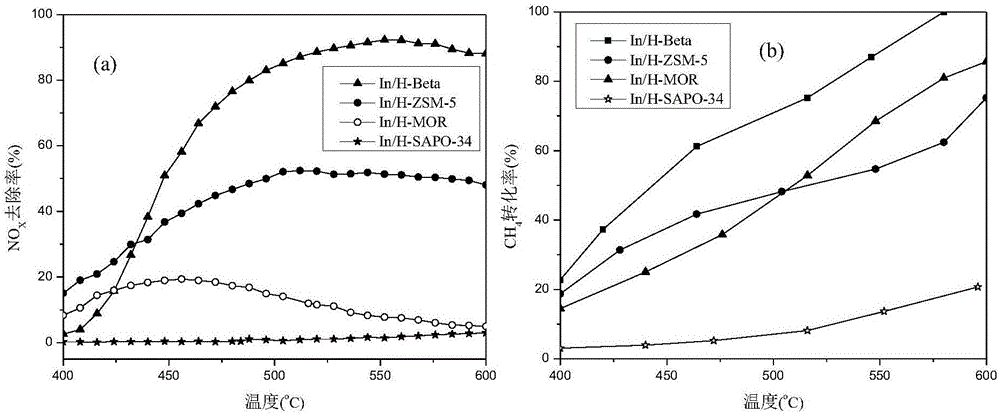
[0064] Investigate the catalysts prepared by different supports to catalyze CH 4 Reduce NO x Reactivity effects, such as figure 1 shown. Depend on figure 1 (a) It can be seen that different carriers have different effects on NO x The influence of the removal rate can be seen that when SAPO-34 is used as the carrier, the catalyst has almost no catalytic activity; the catalytic activity of the catalyst with the MOR carrier is low, and the NO x The removal rate does not exceed 20%; the catalyst with ZSM-5 as the carrier has the best activity in the temperature range below 430 °C, NO x The highest removal rate can reach 50%; the catalyst with H-Beta as the carrier has the highest catalytic activity in the temperature range of 430 ° C ~ 600 ° C, NO x The highest removal rate can reach more than 90%. This result shows that the In / H-Beta molecular sieve catalyst is effective for NO x showed superior catalytic activity. Depend on figure 1 (b) and different carrier pair CH in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com