Synthesis of SBA-15-SO3II solid-phase catalyst and Beckmann rearrangement, esterification catalytic performances
A technology of esterification reaction and solid acid catalyst, which is applied in the direction of catalyst activation/preparation, molecular sieve catalyst, physical/chemical process catalyst, etc. It can solve the problems of cumbersome, unfavorable practical application, and uninspected catalyst catalytic performance, etc., to achieve good catalytic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
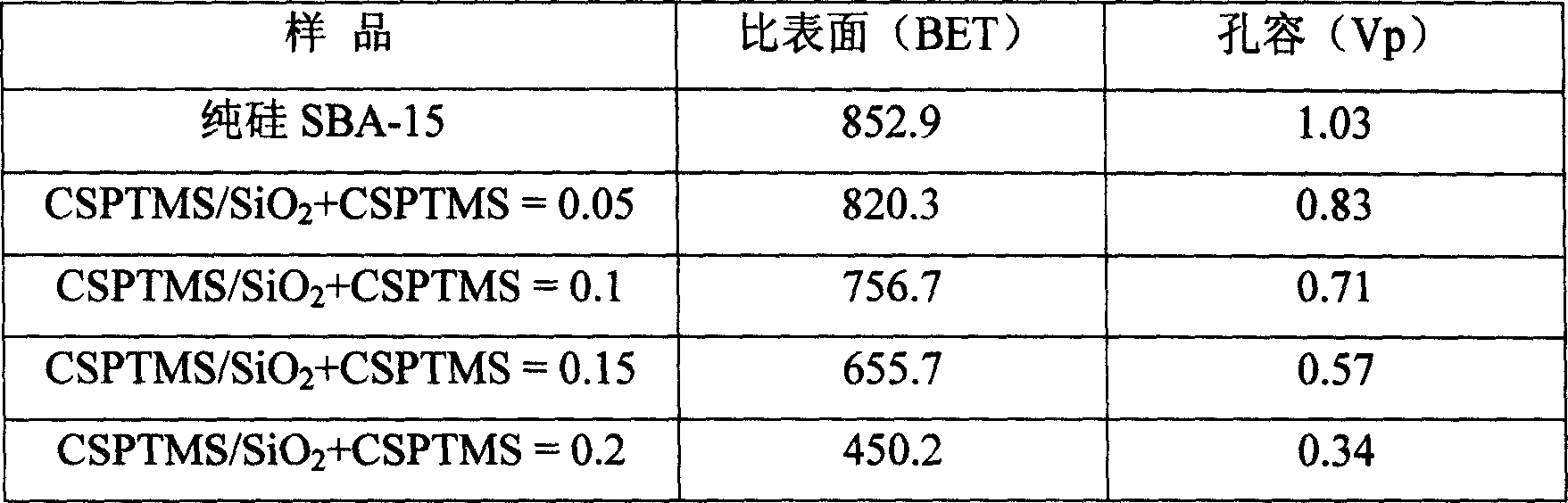
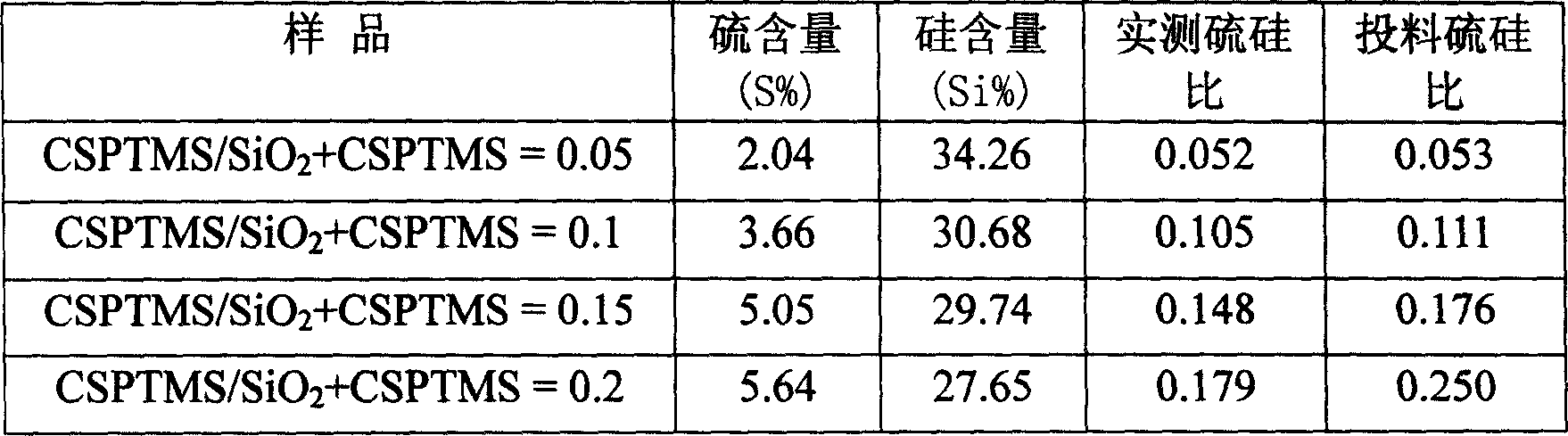
[0030] At a constant stirring speed, add a certain amount of TEOS and CSPTMS dropwise into 4g of P123 hydrochloric acid solution, and stir at 40°C to obtain a molar composition of 1+xSiO 2 :x CSPTMS:6.5HCl:180H 2 A mixture of O where x = 0.05, 0.1, 0.15, 0.2. The mixture was further stirred at 40 °C for 30 h. Then, let it stand at 100°C for 1 day, and the product is suction filtered and dried to obtain the synthetic SBA-15-Ph-SO 3 H. After the sample was extracted with ethanol for 20 hours, it was directly roasted in air at 210°C for 6 hours to obtain the desired catalyst. Sample N 2 The adsorption-desorption isotherm curve, XRD pattern, specific surface area, pore volume and elemental analysis results are shown in Figure 1-Figure 2 and Table 1-Table 2.
example 2
[0032] Preparation of Synthetic SBA-15-Ph-SO 3 The process of H is the same as example 1, and the synthetic type SBA-15-Ph-SO is taken 3 H is directly calcined at 210°C in air for 6 hours to obtain SBA-15-Ph-SO 3 H catalyst
example 3
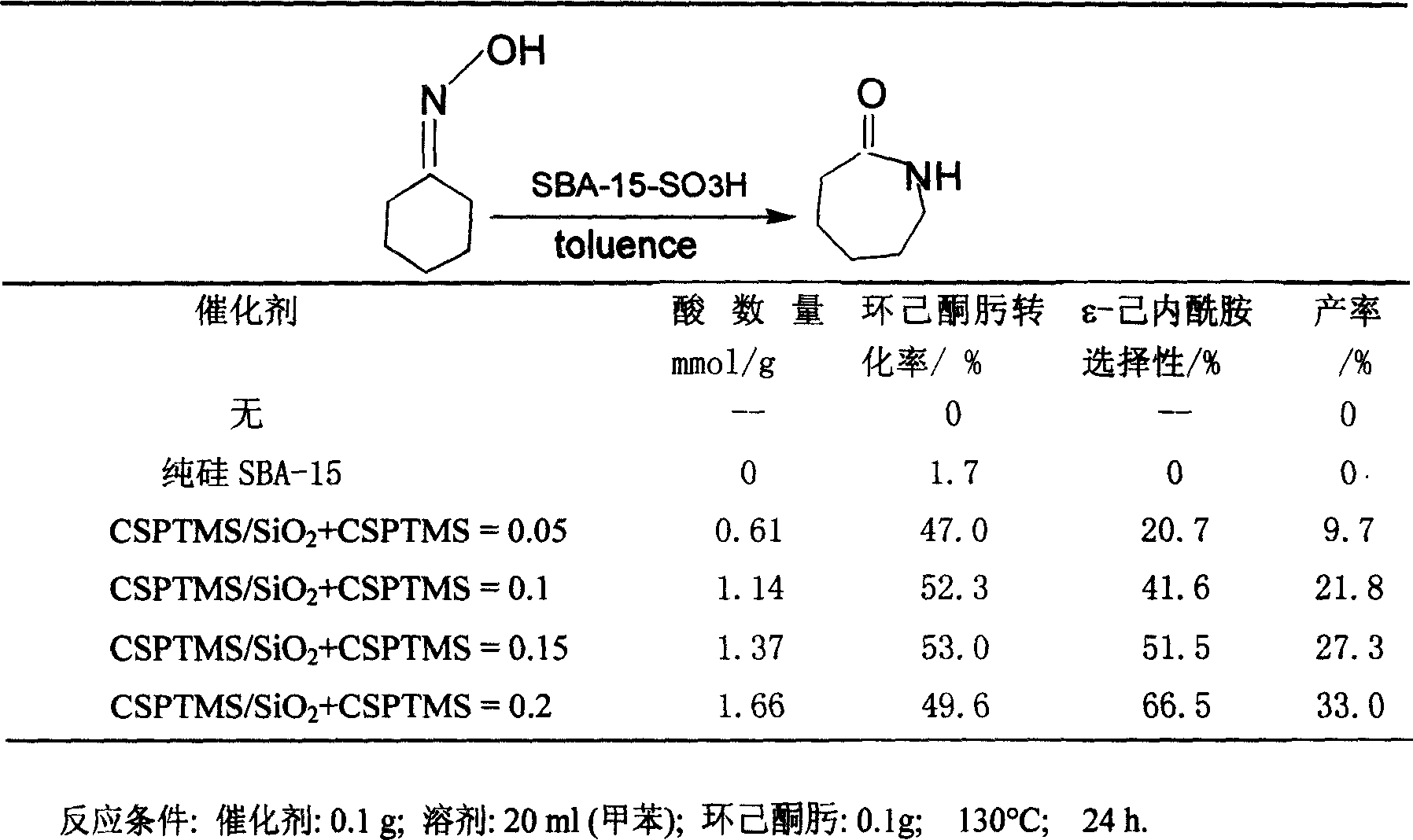
[0034]Add 0.1 g of catalyst, 0.1 g ε-caprolactam, and 20 ml of toluene into a 50 ml two-necked flask equipped with a condenser. After stirring at 130°C for 24 hours in an oil bath, samples were taken and analyzed by GC-MAS (chromatography-mass spectrometry).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com