Method for reducing and removing nitride in metal lithium or lithium alloy
A lithium alloy and metal lithium technology, applied in the field of lithium metal, can solve the problems affecting the quality of metal lithium or alloy, the inability to remove active metal aluminum, and the inability to remove metal lithium, and achieve a short reaction time, low cost, and strong practicability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Nitrogen removal by metal lithium with excessive nitrogen content
[0047] Put 1.51 kg of battery-grade lithium metal with nitrogen content exceeding the standard in a sealed reactor, evacuate the reactor to stabilize it to <0.1Pa, and heat the material at a heating rate of 200°C / h. When the lithium metal is completely melted Stop heating and start stirring to ensure that the nitrogen content of each part of the lithium solution is evenly distributed, and then sample and analyze the nitrogen content in the product according to the national standard analysis method, and the measured nitrogen content is 982ppm. Accurately weighed magnesium-aluminum alloys are added to the molten lithium liquid at a molar ratio of Mg-Al:N=1:1. Reheat the reactor, control the temperature rise rate at 100°C / h, and control the reaction temperature at 300°C, the stirring rate is 300rpm, the reaction time is 0.1h, the forward rotation and the reverse rotation account for half, and aft...
Embodiment 2
[0051] Example 2 Processing Lithium Metal under the Changed Situation of Nitrogen Removal Source
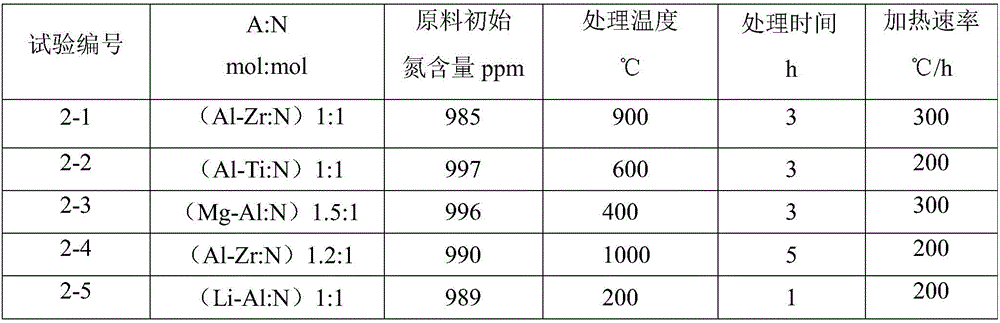
[0052] The experimental method is as described in Example 1. The nitrogen removal source in the experiment was changed to obtain the treatment results under different conditions. These factors are the use of metal aluminum-titanium, aluminum-zirconium alloy or magnesium-aluminum alloy (the shape is alloy foil). The test arrangement is shown in Table 2, and the residual amount of nitrogen and nitrogen removal source in the lithium metal after nitrogen removal and the recovery rate of metal lithium are shown in Table 3.
[0053] Table 2
[0054]
[0055] table 3
[0056] Test No. A content (ppm) N content (ppm) Recovery rate of Li (%) 2-1 (Al-Zr)4 41 98 2-2 (Al-Ti)4 38 99 2-3 (Mg-Al)5 44 99 2-4 (Al-Zr)6 41 98 2-5 (Li-Al)3 40 99
Embodiment 3
[0057] Example 3 Nitrogen removal by lithium aluminum alloy with excessive nitrogen content
[0058] Place 1.78kg of lithium aluminum alloy (standard aluminum content is 3000ppm) with excessive nitrogen content due to damaged packaging in a sealed reactor, feed nitrogen into the reactor, and heat the material under the protection of nitrogen. When the alloy is completely melted, stop heating and start stirring to ensure that the nitrogen content in each part of the lithium liquid is evenly distributed, and then take samples to analyze the nitrogen content and aluminum content in the product according to the industry standard analysis method. The measured nitrogen content is 467ppm , the aluminum content is 2915ppm. Add 1.603 g of accurately weighed aluminum-zirconium alloy grains to the molten lithium liquid at a molar ratio of Al-Zr:N=1:1. Reheat the reactor, control the heating rate at 200°C / h, and control the reaction temperature at 600°C, the stirring rate is 300rpm, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com