I-group 4-type fowl adenovirus DNA vaccine and application thereof
A DNA vaccine, avian adenovirus technology, applied in recombinant DNA technology, antiviral agents, medical preparations containing active ingredients, etc. The effect of chemical development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
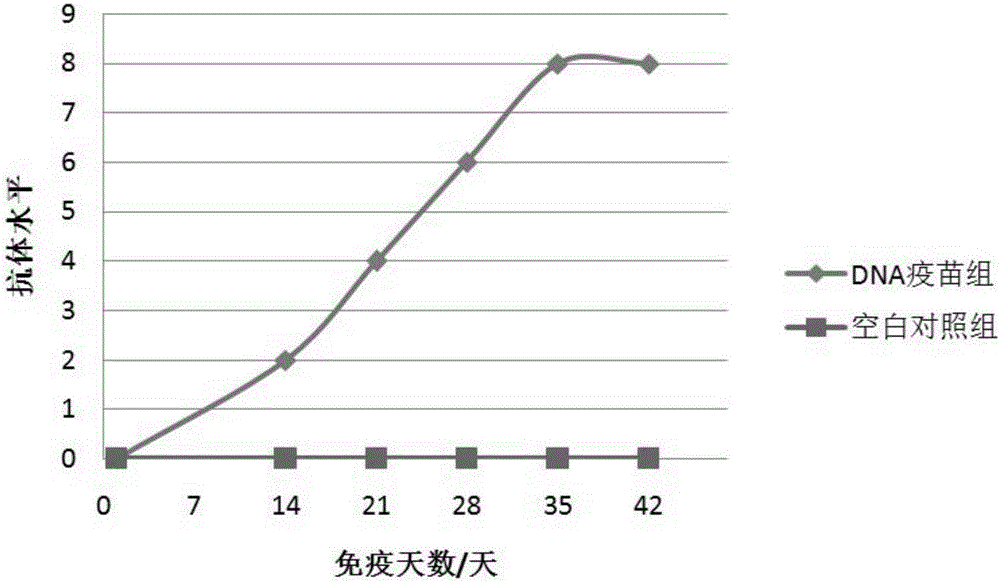
Image
Examples
Embodiment 1
[0026] 1.1 Fibrin C-terminal gene cloning and sequence analysis:
[0027] Design and synthesize a pair of primers:
[0028] Primer 1: 5'AAACATATGTCGAGCTCGGTACCTTT 3';
[0029] Primer 2: 5'AAACTCGAGTTATGTAAAGGGAATGG 3'.
[0030] The virulent strain of FAV-4 was isolated from diseased broilers.
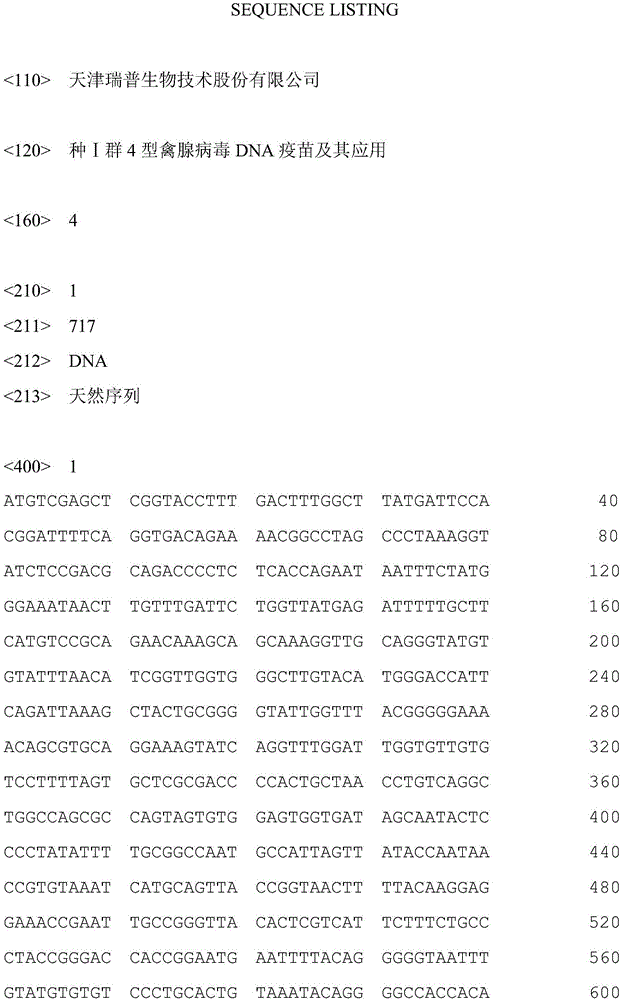
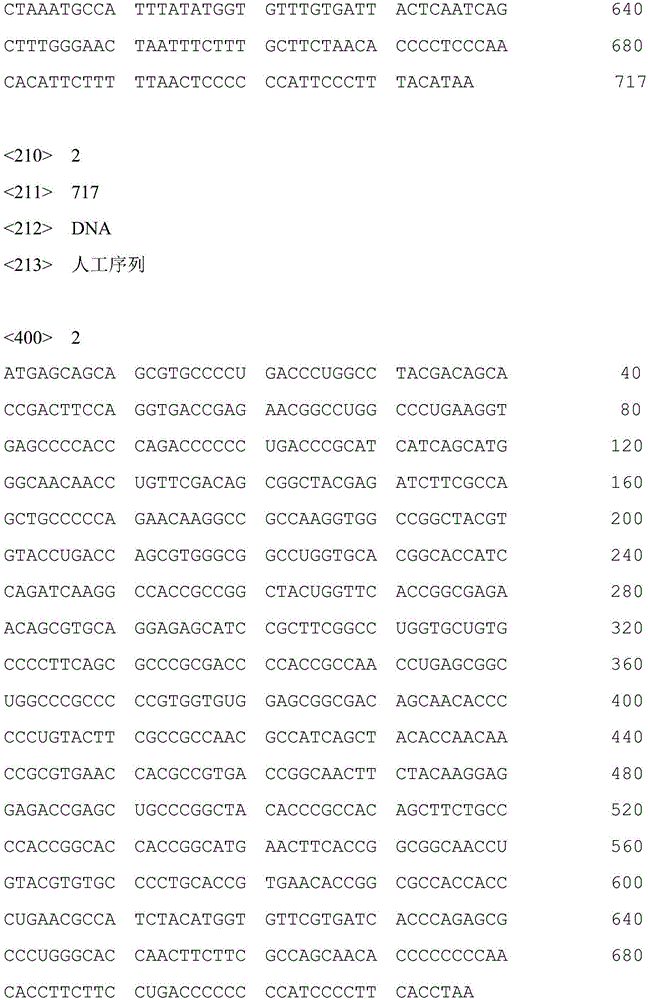
[0031] Using the FAV-4 virus as a template, denaturation at 94°C for 45s, annealing at 56°C for 45s, and extension at 72°C for 1min, a total of 30 cycles of PCR amplification were performed; the PCR product was directly cloned into the pMD-18T vector (TaKaRa Company) to construct pMD-18T-FAV4C: Sequencing was carried out by the dideoxy chain termination method, and the results showed that the gene encoding the C-terminus of fibrin cloned from the FAV-4 genome was shown in SEQ ID No.1. The gene was codon-optimized according to the codon preference of chickens, and the optimized FAV-4-C gene was shown in SEQ ID No.2, and the gene was synthesized onto the pMD-18T vector to construct pMD...
Embodiment 2
[0042] A DNA vaccine of group I type 4 avian adenovirus, the antigen of the vaccine contains the C-terminal expression gene of fibrin of group I type 4 adenovirus.
[0043] On the basis of the above technical solutions, the following conditions are met:
[0044] The C-terminal expression gene of the fibrin protein of group I type 4 avian adenovirus is a product obtained by replacing chicken rare codons with chicken biased codons on the basis of the natural expression genes.
Embodiment 3
[0046] A DNA vaccine of group I type 4 avian adenovirus, the antigen of the vaccine contains the C-terminal expression gene of fibrin of group I type 4 adenovirus.
[0047] On the basis of the above technical solutions, the following conditions are met:
[0048] The expression gene at the C-terminal end of the fibrin of the group I group 4 avian adenovirus is the DNA whose sequence is shown in SEQ ID No.2.
[0049] The antigen is prepared by the following method:
[0050] 1) Using the DNA fragments shown in SEQ ID No.3 and SEQ ID No.4 as primers and FAV-4 virus as a template, denature at 94°C for 45s, anneal at 56°C for 45s, and extend at 72°C for 1min for a total of 30 PCR amplification is carried out in cycles; the PCR product is directly cloned into the pMD-18T vector to obtain the recombinant vector pMD-18T-FAV4C;
[0051] 2) Optimizing the nucleotide sequence of the FAV-4-C gene to the state shown in SEQ ID No.2, and synthesizing the gene into the pMD-18T vector to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com