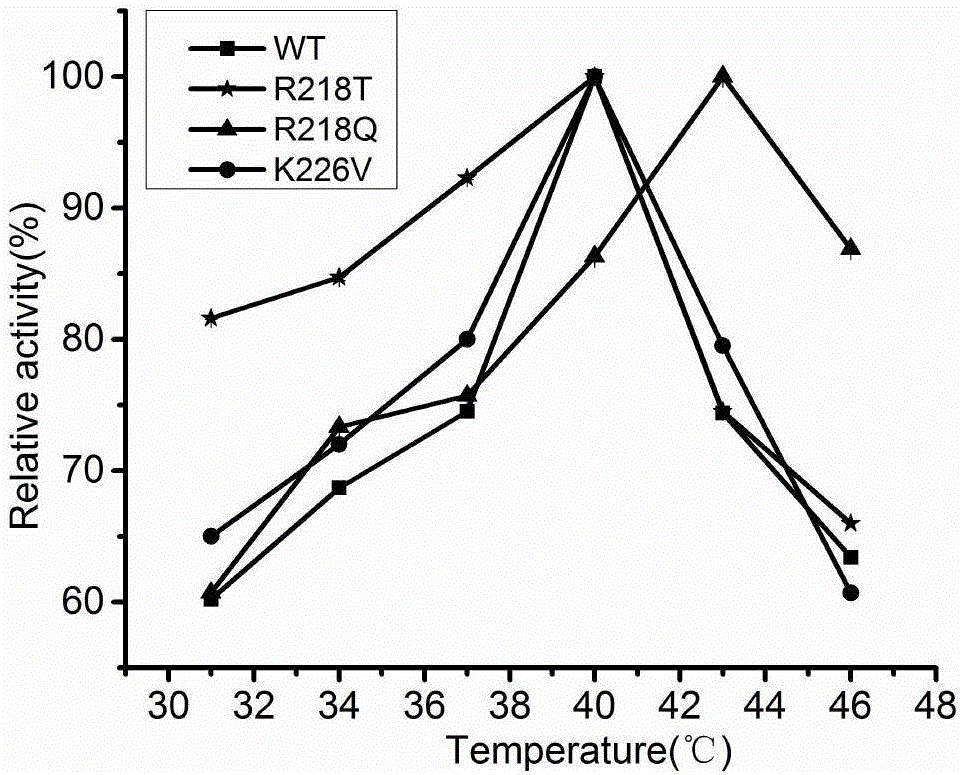
Cephalosporin C acylase mutant with higher heat stability and construction method thereof
A cephalosporin and thermal stability technology, applied in the field of biological genetic engineering, can solve the problems of reduced catalytic efficiency, CPC enzyme inactivation, poor stability, etc., achieve good reaction temperature, good thermal stability, and ensure production catalysis efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Cloning of embodiment 1 wild-type cephalosporin C acylase gene
[0041] The cephalosporin C acylase gene derived from Pseudomonas sp.SE83 was codon-optimized with Escherichia coli as the host, and the sequences of the upstream and downstream primers were designed as follows:
[0042] Upstream primer F: 5'-ATAT CATATG ACGATGGCGGCCAAGACCGATCGCGAGGCCCTGCAGGCGGCGCTGCCGCCGCTTTCCGGCAGCCTCTCCATTCCGGGTTTAAGCGCCCCTG-3'
[0043] Downstream primer R: 5'-ATAT CTCGAG TTAGGCCGGAACCAGCTCCTGGCTG-3'
[0044] Wherein the underlines are NdeI and XhoI restriction sites respectively. PCR reaction system: 25 μL of 2×PrimeStar Max DNA polymerase, 1.0 μL of upstream and downstream primers (10 μmol / L), 1.0 μL of gene template (50 ng / μL), and 22 μL of double distilled water. The PCR reaction conditions were: 98°C for 2min, then 98°C for 10sec, 55°C for 15sec, 72°C for 30sec, a total of 25 cycles; and finally 72°C for 10min. After the reaction, the PCR amplification product was detected by...
Embodiment 2
[0045] Example 2 Expression and purification of wild-type cephalosporin C acylase
[0046] The engineered bacteria were inoculated into 4 mL LB medium test tubes containing 100 μg / mL Kan at a volume ratio of 1%, and cultured at 37°C and 220 rpm for 12 hours. Transfer the 4mL bacterial liquid to a 1L LB medium shake flask containing 50μg / mL Kan, culture at 37°C 220rpm for about 2.5h, make the OD600 reach about 0.9, add 0.5mM IPTG inducer, and induce culture at 25°C 220rpm for 12- 16h. The Escherichia coli cell suspension harvested after fermentation is ultrasonically disrupted, and then subjected to Ni-NTA affinity chromatography to obtain the target protein with a purity of more than 95%.
Embodiment 3
[0047] Example 3 Homologous modeling of wild-type cephalosporin C acylase
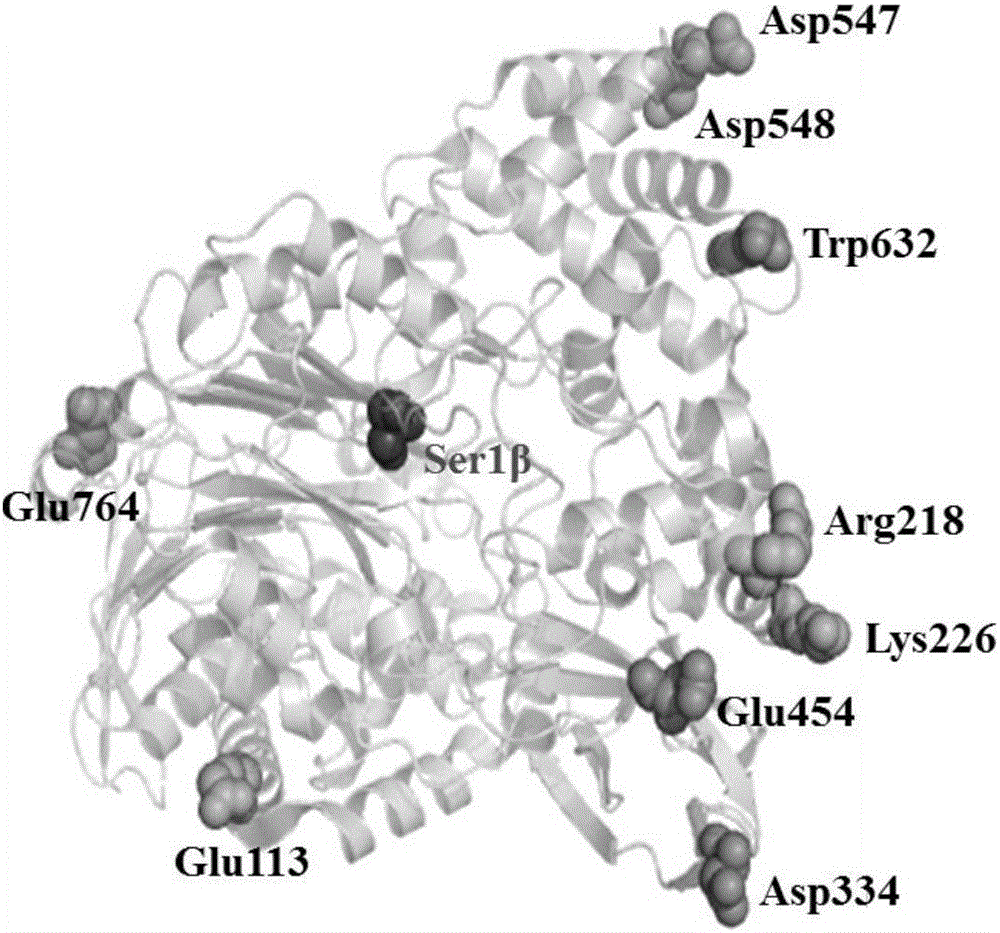
[0048] Since the crystal structure of wild-type cephalosporin C acylase SE83acyII S12 has not yet been resolved, it is difficult to operate through conventional protein rational design methods, so we provide a homology model using N176acy with high homology (PDB ID: 4HSR) as a template Methods.
[0049] 1) Enter the homepage of the SWISS-MODEL database (https: / / www.swissmodel.expasy.org / ), enter the amino acid sequence of cephalosporin C acylase SE83acyII S12 in the Target Sequence tool to search. The server directly searches for the sequence with the highest homology 4HSR as a template for sequence comparison, and obtains the structure model of the target protein.
[0050] 2) Search for the structure of cephalosporin C acylase (N176acy, PDB ID: 4HSR) with the highest homology to cephalosporin C acylase SE83acyII S12 derived from Pseudomonas diminutaN176 in the Protein Data Bank (PDB database), The s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com