Detection kit for listeria monocytogenes in food and application method
A technique for monocytogenes and Listeria, applied in the biological field, can solve the problems of inability to truly reflect the level of sample contamination, cumbersome and time-consuming operation, low sensitivity, etc., and achieve the effect of saving detection time, high sensitivity, and low background interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In this embodiment, the sensitivity test of the micro-gap array electrode kit with 96 holes is performed according to the following steps:
[0029] (1) Preparation of Listeria monocytogenes suspension: heat the bacterial solution in a water bath at 100°C for 15 minutes, and then perform serial dilutions to 10 8 cfu / mL, 10 7 cfu / mL, 10 6 cfu / mL, 10 5 cfu / mL, 10 4 Cfu / mL concentration of bacterial liquid;
[0030] (2) Drop the bacterial liquid into the chip hole embedded with the polyclonal antibody of Listeria monocytogenes, obtain the conductivity signal related to the concentration of Listeria monocytogenes from the electrode, and record the change value ,Such as figure 2 Shown.
[0031] The results showed that the higher the concentration of Listeria monocytogenes, the greater the conductance value, and the minimum detection limit can reach 10 5 cfu / mL.
Embodiment 2
[0033] In this embodiment, a specific test is performed on a 96-hole microgap array electrode kit, which is specifically performed according to the following steps:
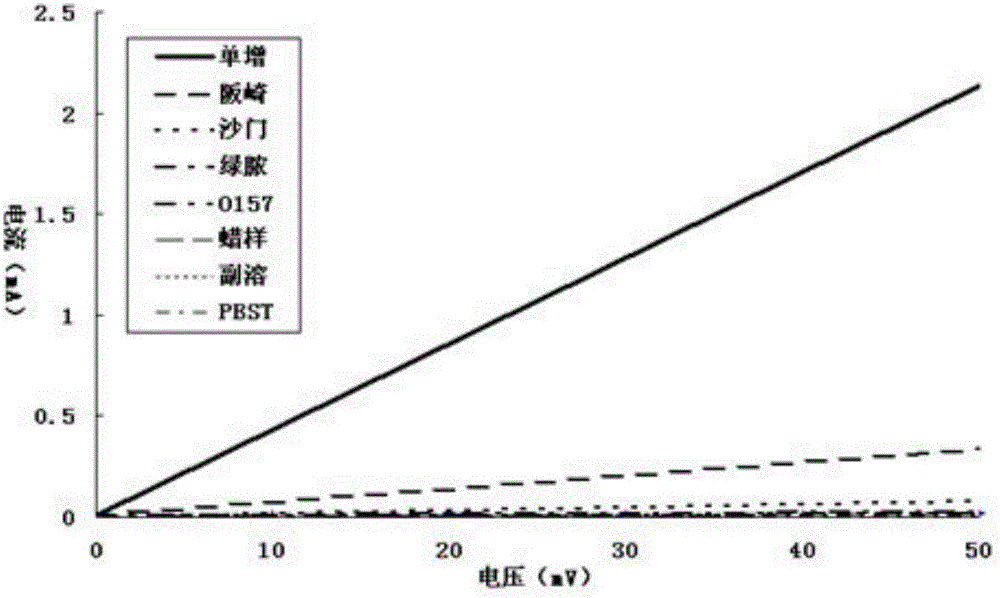
[0034] Listeria monocytogenes, Enterobacter sakazakii, Salmonella, Pseudomonas aeruginosa, Escherichia coli O157:H7, Bacillus cereus, Vibrio parahaemolyticus (concentration of 10 7 cfu / mL) as the sample to be tested for cross-reaction research. The reaction results of the kit are as follows image 3 As shown, only Listeria monocytogenes is positive, and the rest are negative, indicating that the kit has good specificity.
Embodiment 3
[0036] The analysis of the actual sample using the 96-well microgap array electrode kit of this embodiment is carried out according to the following steps:
[0037] Take a positive sample of Listeria monocytogenes, and refer to the national standard GB 4789.30-2010 for sample processing, that is, take 25g of the test sample aseptically and add it to LB containing 225mL Listeria broth 1 In the homogenization cup of the enrichment liquid, homogenize with a homogenizer for 1 min to prepare a 1:10 sample homogenate, and incubate at 30℃±1℃ for 24h. Pipette 0.1mL of liquid and transfer to 10mL of Listeria broth LB 2 In the enrichment solution, incubate at 30°C±1°C for 18-24 hours, take 1 mL of the bacterial solution into a small test tube, heat it in a water bath at 100°C for 15 minutes, take it out, and cool to room temperature as the sample to be tested.
[0038] The sample to be tested is set up with positive and negative controls at the same time, and the test is performed. The reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com