Novel method used for synthesizing cholesterol
A technology of cholesterol and new method, applied in the field of synthesizing cholesterol, can solve the problems of large consumption of raw and auxiliary materials, uneconomical, long steps, etc., and achieve the effects of reducing unit consumption, low cost and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
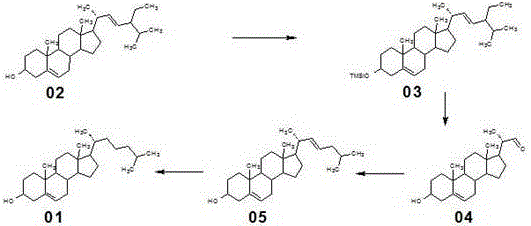
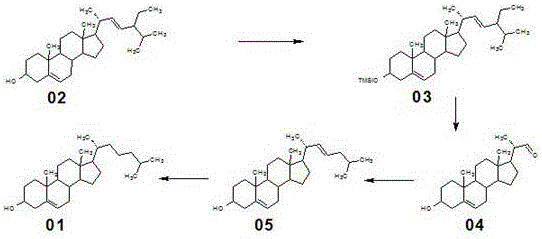
[0030] A new method for synthesizing cholesterol, comprising the following steps:
[0031] (1) At room temperature, add 1000ml of dichloromethane to a 2000L reaction glass reaction bottle, stir, add 37g of imidazole, add 200g of stigmasterol 02, cool down in an ice-water bath to below 10°C, slowly add 59g of trimethylchlorosilane dropwise, After the addition was completed, the internal temperature was controlled at 20°C and the reaction was incubated for 1.5 hours. After the reaction, wash with water until neutral, add 400ml of tap water, concentrate at normal pressure until there is no solvent smell, cool down to below 40°C, filter, wash with water, drain, and dry to obtain 229.21g of compound 03 with a molar yield of 97.36%.
[0032] (2) Dissolve 20 g of compound 03 in 1000 mL of dichloromethane, cool down to -70 °C, slowly bubble in ozone, react for 2 hours, the reaction is complete, the reaction is moved to room temperature, add 18.4 g of zinc powder and 20 mL of Glacial ...
Embodiment 2
[0036] The synthesis method is the same as in Example 1, the only difference is that in this example, the amount of imidazole and trimethylchlorosilane in step (1) of Example 1 were adjusted to 40g and 64g respectively to obtain 229.05g of compound 03, with a molar yield 97.29%; the amount of zinc powder in step (2) was adjusted to 21.5g, and 11.56g of compound 04 was obtained, with a molar yield of 84.95%; triphenylphosphine, 1-chloro-3-methylbutyl The consumption of alkane, potassium tert-butoxide, and 30% industrial concentrated hydrochloric acid are adjusted to 94g, 39g, 48g, and 58g respectively to obtain 55.05g compound 05, with a molar yield of 90.98%; 1% palladium carbon catalyst in step (4) and ammonium acetate were adjusted to 4g and 8g, respectively, to obtain 184.21g of cholesterol 01, and the molar yield was 91.63%.
Embodiment 3
[0038]The synthesis method is the same as in Example 1, the only difference is that in this example, the amount of imidazole and trimethylchlorosilane in step (1) of Example 1 were adjusted to 43g and 69g respectively, and 228.34g of compound 03 was obtained, with a molar yield 96.99%; the amount of zinc powder in step (2) was adjusted to 24.5g, and 11.50g of compound 04 was obtained, with a molar yield of 84.51%; triphenylphosphine, 1-chloro-3-methylbutane in step (3) , Potassium tert-butoxide consumption is adjusted to be 125g, 58g and 42.5g respectively, and 30% industrial concentrated hydrochloric acid is changed into the industrial concentrated sulfuric acid of 32g, obtains 54.39g compound 05, molar yield 90.78%; 1 in step (4) % palladium carbon catalyst and ammonium acetate were adjusted to 6g and 12g respectively, to obtain 184.05g cholesterol 01, and the molar yield was 91.55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com