Method for preparing benzopyrone compound through adopting pentacarbonyl iron as CO release source
A technology of benzopyrone and iron pentacarbonyl, applied in the direction of organic chemistry, etc., to achieve the effect of reducing the harsh requirements of reaction equipment, easy control, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
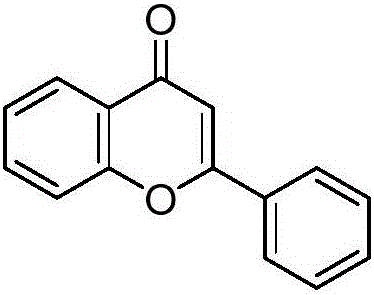
[0012] Preparation of 2-phenyl-4H-benzopyran-4-one with the following structural formula
[0013]
[0014] 0.11g (0.5mmol) 2-iodophenol, 72μL (0.75mmol) phenylacetylene, 0.0056g (0.025mmol) palladium acetate, 0.1293g (1.5mmol) piperazine and 30μL (0.15mmol) pentacarbonyl iron, 3mL anhydrous Add acetonitrile into the Shrek tube, stir and react at 50°C for 12 hours, stop the reaction, cool down to room temperature naturally, and separate by column chromatography to obtain a yellow solid 2-phenyl-4H-benzopyran-4-one with a yield of 95%, the structural characterization data is: 1 H NMR (400MHz, CDCl 3 )δ8.24(d,J=9.5Hz,1H),7.93(d,J=8.0Hz,2H),7.74-7.67(m,1H),7.56(dd,J=18.1,7.0Hz,4H), 7.43(t, J=7.5Hz, 1H), 6.84(s, 1H); 13 C NMR (101MHz, CDCl 3 )δ 178.63, 163.60, 156.45, 133.93, 131.98, 131.76, 129.20, 126.47, 125.89, 125.39, 124.15, 118.24, 107.78, 100.13.
Embodiment 2
[0016] Preparation of 2-(4-methoxyphenyl)-4H-benzopyran-4-one with the following structural formula
[0017]
[0018] In Example 1, the phenylacetylene used was replaced with equimolar 4-methoxyphenylacetylene, and other steps were the same as in Example 1 to obtain a yellow solid 2-(4-methoxyphenyl)-4H-benzopyridine Furan-4-one, its yield is 94%, and structural characterization data is: 1 H NMR (400MHz, CDCl 3 )δ8.23(d, J=8.0Hz, 1H), 7.89(d, J=8.8Hz, 2H), 7.68(t, J=7.6Hz, 1H), 7.54(s, 1H), 7.41(t, J=7.5Hz, 1H), 7.03(d, J=8.7Hz, 2H), 6.75(s, 1H), 3.89(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ 178.68, 163.92, 162.71, 156.72, 133.70, 128.16, 125.83, 125.23, 124.21, 124.11, 118.10, 114.63, 106.36, 100.13, 55.65.
Embodiment 3
[0020] Preparation of 2-(4-tolyl)-4H-benzopyran-4-one with the following structural formula
[0021]
[0022] In Example 1, the phenylacetylene used was replaced with an equimolar 4-methylphenylacetylene, and other steps were the same as in Example 1 to obtain a yellow solid 2-(4-methylphenyl)-4H-benzopyran-4- Ketone, its yield is 94%, and structural characterization data is: 1 HNMR (400MHz, CDCl 3 )δ8.22(d, J=7.6Hz, 1H), 7.82(d, J=8.1Hz, 2H), 7.68(t, J=8.0Hz, 1H), 7.55(d, J=8.4Hz, 1H) ,7.41(t,J=7.5Hz,1H),7.31(d,J=8.1Hz,2H),6.79(s,1H),2.43(s,3H); 13 C NMR (101MHz, CDCl 3 ) δ 178.61, 163.75, 156.37, 142.38, 133.78, 129.89, 129.07, 126.35, 125.80, 125.25, 124.11, 118.17, 107.09, 21.65.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com