Metal organic frame based on pyridine oxide ligand, and preparation method and application thereof
A metal-organic framework, pyridine oxidation technology, applied in the directions of organic chemistry methods, preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of cumbersome preparation steps, unrecoverable, poor stability, etc., and achieves cost reduction, easy recovery, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Preparation of oxidized pyridine ligand L
[0056] Concrete preparation steps are as follows:
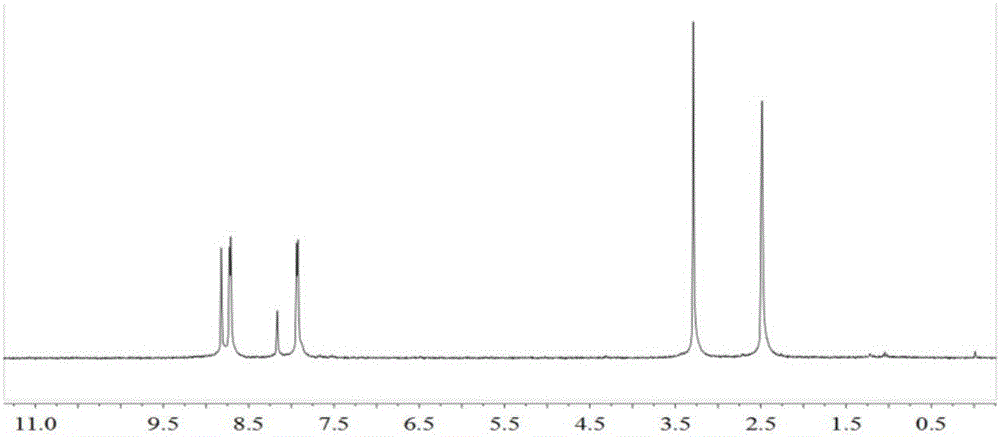
[0057] (1) In a 250mL single-necked bottle, dissolve 3,5-dibromopyridine (3.2g, 13.5mmol) in 50mL THF, add m-chloroperoxybenzoic acid (6.88g, 40mmol), stir at room temperature for 48 hours, and the reaction ends Then add 30mL saturated aqueous sodium carbonate solution, let stand to separate layers, and use 2×50mL CH for the aqueous phase 2 Cl 2 Extract, combine the organic phase and wash with anhydrous Na 2 SO 4 Drying, silica gel column chromatography (CH2 Cl 2 ) to obtain 1.53 g of light white solid, namely 3,5-dibromopyridine-N-oxide, with a yield of 44.1%. 1 H NMR (300MHz, DMSO, 25°C, TMS): δ=8.60(s, 2H, -C 5 h 3 NBr 2 ),7.97(s,1H,-C 5 h 3 NBr 2 ).
[0058]
[0059] (2)N 2 Under protection, 3,5-dibromopyridine-N-oxide (2.53g, 10.0mmol), 4-pyridineboronic acid (2.95g, 24.0mmol), tetrakistriphenylphosphine palladium (1.16g, 1.0mmol), Anhydrou...
Embodiment 2
[0062] Embodiment 2: the synthesis of Zn-MOF
[0063] At room temperature, the ZnBr 2 (2.2mg, 0.01mmol) was dissolved in methanol (2mL) to obtain ZnBr 2 methanol solution, L (2.4 mg, 0.01 mmol) was dissolved in CH 2 Cl 2 solution (2mL), the methanol solution was slowly spread over CH 2 Cl 2 On the solution, a colorless single crystal was obtained after standing for three days.
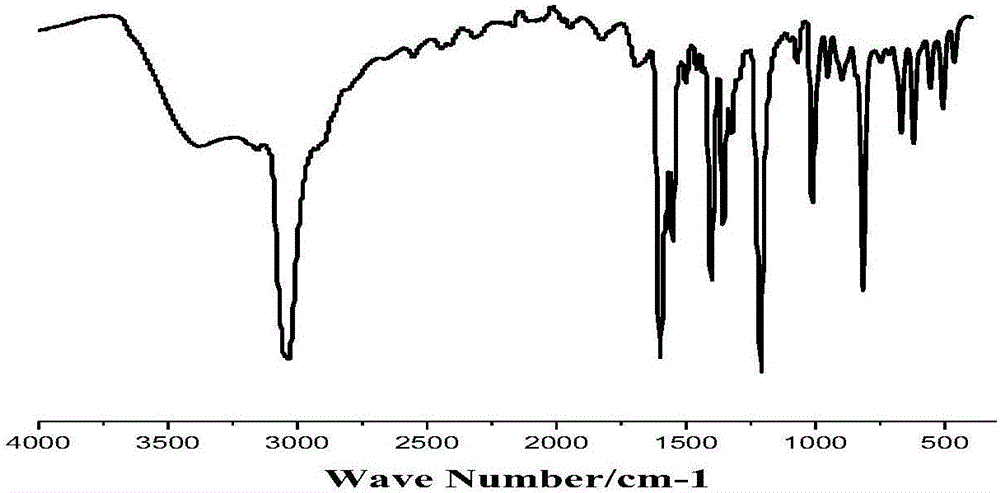
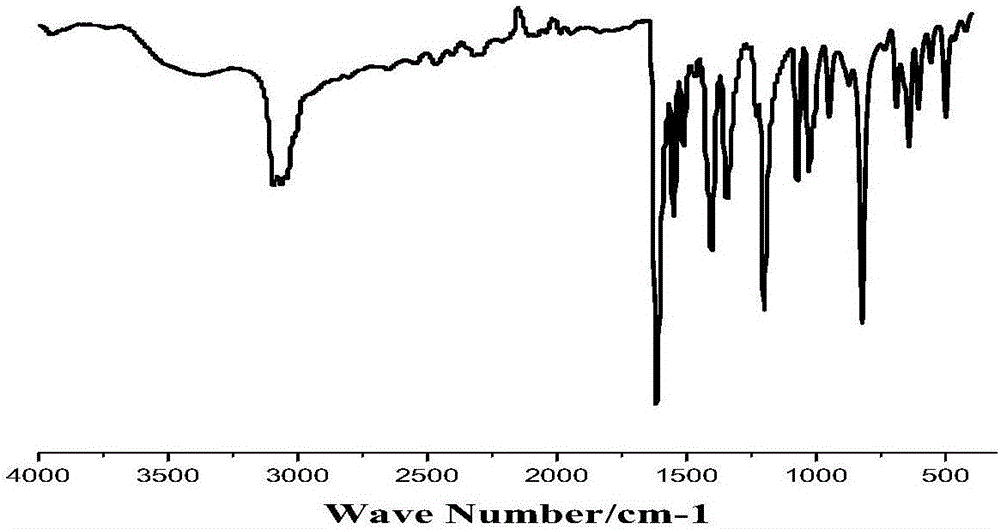
[0064] By infrared spectrogram (IR), thermogravimetric analysis spectrogram (TGA) has characterized this compound, and the results are shown in image 3 , Figure 4 .
[0065] Zn-MOF single crystal structure such as Figure 5 shown.
[0066] Depend on Figure 5 It can be seen that Zn-MOF is monoclinic and belongs to the P2(1) / n space group. There is only one kind of Zn(II) center in Zn-MOF, which is in (ZnN 2 Br 2 ) in the coordination environment, the nitrogen atom coordinated with it is from the end group pyridine of the ligand L, and the lengths of the Zn-N bonds are respectively with...
experiment example 3
[0069] Experimental Example 3: Formation of benzaldehyde and malononitrile condensation reaction
[0070] At room temperature, add benzaldehyde (1mmol) and malononitrile (1.2mmol) into a 2mL glass bottle, after stirring for 5 minutes, add the catalyst Zn-MOF (19mg, 4% (mol), based on the amount of benzaldehyde) , continue to stir for 6 hours, and track the reaction with gas chromatography. After the reaction, quickly centrifuge. The solid after centrifugation is the catalyst Zn-MOF for the catalytic reaction. The catalyst is recovered and directly put into the next cycle reaction. According to the above conditions, the catalyst is used 5 cycles, the reaction solution calculates the productive rate by gas chromatography, and the catalytic effect is as shown in table 2, and the catalyst of recovery is characterized by PXRD, as Image 6 shown.
[0071] Table 2 Yield and TOF value of 5 cycles of Zn-MOF catalyzed Knoevenagel condensation reaction
[0072]
[0073] a: The yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com