7-ethyl-10-hydroxycamptothecine-polymer conjugated drug and preparation method of drug nano-preparation
A technology of hydroxycamptothecin and nano-formulation, which is applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of low bioavailability, low conversion rate, loss of activity, etc., and achieve high safety and reduce inactivation. , the effect of slowing drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Synthesis of SN38-polylactic acid coupled prodrug 1 ( figure 1 )
[0053] Add mPLA(600)-SA (207mg, 0.26mmol) and SN38 (100mg, 0.26mmol) into a 100mL round bottom flask, dissolve in 10mL of anhydrous dichloromethane, and add EDC (59mg, 0.38mmol). Stir at 50°C for 4 hours, remove the reaction solvent, and wash with 5% citric acid, saturated sodium bicarbonate, and saturated brine, respectively. The organic phase was dried over anhydrous sodium sulfate, filtered, the filtrate was collected and the solvent was removed under reduced pressure. Product 1 (102 mg, 34%) was obtained after separation and purification by column chromatography (DCM:MeOH=120:1).
[0054] Prodrug 1 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0055] 1 H NMR (400MHz, CDCl 3 ): δ1.00-1.01(t,3H),1.38-1.42(t,3H),1.58(s,24H),1.88-1.92(q,2H),2.89-2.92(t,2H),2.98-3.03 (m,2H),3.13-3.19(q,2H),3.38(s,3H),3.53-3.56(t,2H),3.61-3.64(t,2H),3.68-3.70(t,2H),4.25 -4.33...
Embodiment 2
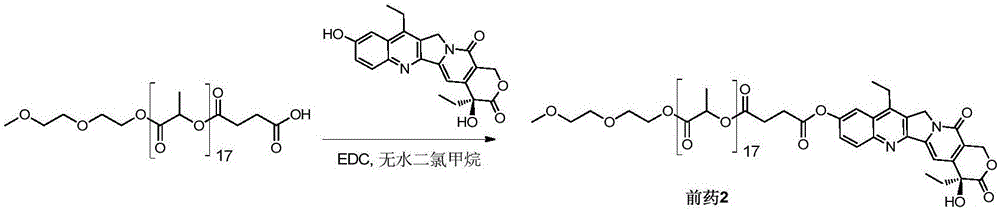
[0056] Example 2 Synthesis of SN38-polylactic acid coupled prodrug 2 ( figure 2 )
[0057] Add mPLA(1200)-SA (357mg, 0.26mmol) and SN38 (100mg, 0.26mmol) into a 100mL round bottom flask, dissolve in 13mL of anhydrous dichloromethane, and add EDC (59mg, 0.38mmol). Stir at 50°C for 4 hours, remove the reaction solvent, and wash with 5% citric acid, saturated sodium bicarbonate, and saturated brine, respectively. The organic phase was dried over anhydrous sodium sulfate, filtered, the filtrate was collected and the solvent was removed under reduced pressure. Product 2 (185 mg, 41%) was obtained after separation and purification by column chromatography (DCM:MeOH=120:1).
[0058] Prodrug 2 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0059] 1 HNMR (400MHz, CDCl 3 ):δ1.02-1.06(t,3H),1.38-1.42(t,3H),1.57-1.61(m,51H),1.88-1.92(q,2H),2.89-2.94(m,2H),2.98 -3.07(m,2H),3.13-3.18(q,2H),3.38(s,3H),3.54-3.56(q,2H),3.63-3.65(q,2H),3.68-3.70(t,2H) ,4.25-...
Embodiment 3
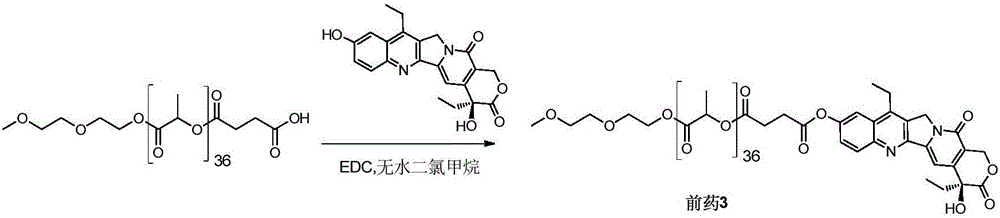
[0060] Example 3 Synthesis of SN38-polylactic acid coupled prodrug 3 ( image 3 )
[0061] Add mPLA(2600)-SA (591 mg, 0.20 mmol) and SN38 (79 mg, 0.20 mmol) into a 100 mL round bottom flask, dissolve in 15 mL of anhydrous dichloromethane, and add EDC (47 mg, 0.30 mmol). Stir at 50°C for 4 hours, remove the reaction solvent, and wash with 5% citric acid, saturated sodium bicarbonate, and saturated brine, respectively. The organic phase was dried over anhydrous sodium sulfate, filtered, the filtrate was collected and the solvent was removed under reduced pressure. Product 3 (320 mg, 48%) was obtained after separation and purification by column chromatography (DCM:MeOH=120:1).
[0062] Prodrug 3 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0063] 1 HNMR (400MHz, CDCl 3 ):δ1.02-1.06(t,3H),1.38-1.42(t,3H),1.53-1.61(m,108H),1.86-1.92(m,2H),2.89-2.92(m,2H),2.98 -3.03(m,2H),3.13-3.19(q,2H),3.38(s,3H),3.53-3.56(q,2H),3.63-3.65(t,2H),3.68-3.70(t,2H) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com