7-Ethyl-10-hydroxycamptothecin-polymer conjugated drug and preparation method of nano preparation
A technology of hydroxycamptothecin and nano preparations, which is applied in drug combination, drug delivery, and pharmaceutical formulations, etc. It can solve problems such as low bioavailability, low conversion rate, and large side effects, and achieve high safety and reduced inactivation , the effect of slowing down the drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
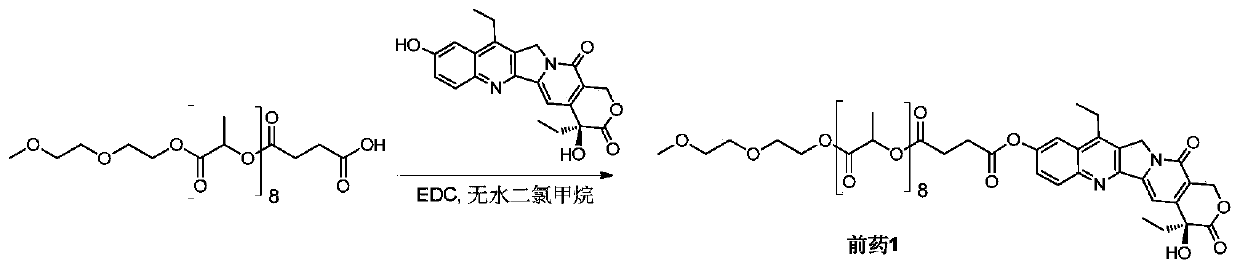
[0052] Example 1 Synthesis of SN38-polylactic acid coupled prodrug 1 ( figure 1 )
[0053] Add mPLA(600)-SA (207mg, 0.26mmol) and SN38 (100mg, 0.26mmol) into a 100mL round bottom flask, dissolve in 10mL of anhydrous dichloromethane, and add EDC (59mg, 0.38mmol). Stir at 50°C for 4 hours, remove the reaction solvent, and wash with 5% citric acid, saturated sodium bicarbonate, and saturated brine, respectively. The organic phase was dried over anhydrous sodium sulfate, filtered, the filtrate was collected and the solvent was removed under reduced pressure. Product 1 (102 mg, 34%) was obtained after separation and purification by column chromatography (DCM:MeOH=120:1).
[0054] Prodrug 1 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0055] 1 H NMR (400MHz, CDCl 3 ): δ1.00-1.01(t,3H),1.38-1.42(t,3H),1.58(s,24H),1.88-1.92(q,2H),2.89-2.92(t,2H),2.98-3.03 (m,2H),3.13-3.19(q,2H),3.38(s,3H),3.53-3.56(t,2H),3.61-3.64(t,2H),3.68-3.70(t,2H),4.25 -4.33...
Embodiment 2
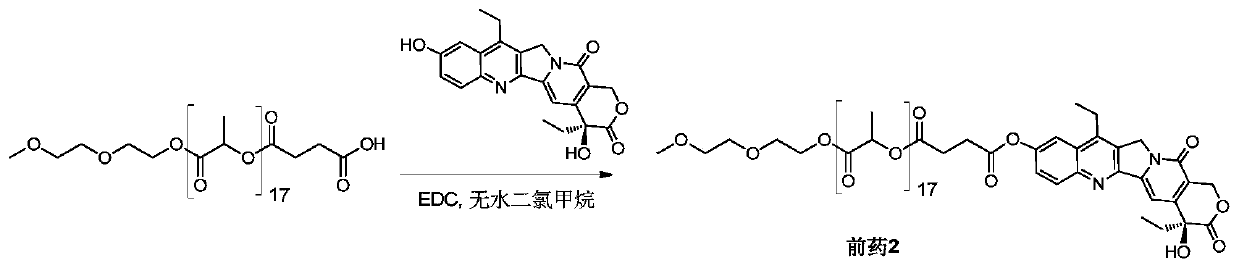
[0056] Example 2 Synthesis of SN38-polylactic acid coupled prodrug 2 ( figure 2 )
[0057] Add mPLA(1200)-SA (357mg, 0.26mmol) and SN38 (100mg, 0.26mmol) into a 100mL round bottom flask, dissolve in 13mL of anhydrous dichloromethane, and add EDC (59mg, 0.38mmol). Stir at 50°C for 4 hours, remove the reaction solvent, and wash with 5% citric acid, saturated sodium bicarbonate, and saturated brine, respectively. The organic phase was dried over anhydrous sodium sulfate, filtered, the filtrate was collected and the solvent was removed under reduced pressure. Product 2 (185 mg, 41%) was obtained after separation and purification by column chromatography (DCM:MeOH=120:1).
[0058] Prodrug 2 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0059] 1 HNMR (400MHz, CDCl 3 ):δ1.02-1.06(t,3H),1.38-1.42(t,3H),1.57-1.61(m,51H),1.88-1.92(q,2H),2.89-2.94(m,2H),2.98 -3.07(m,2H),3.13-3.18(q,2H),3.38(s,3H),3.54-3.56(q,2H),3.63-3.65(q,2H),3.68-3.70(t,2H) ,4.25-...
Embodiment 3
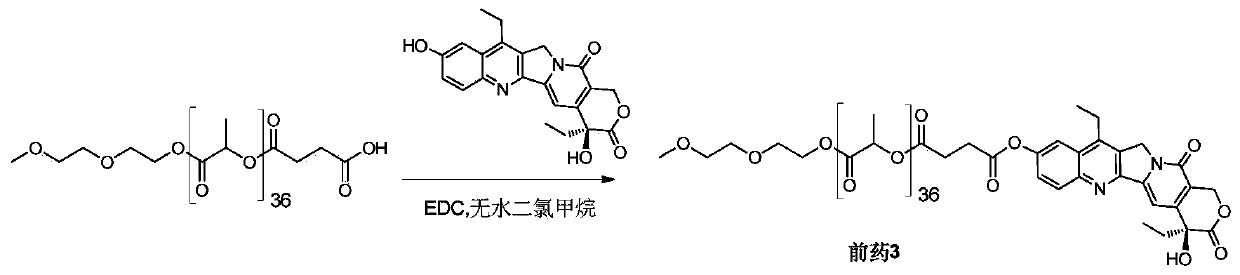
[0060] Example 3 Synthesis of SN38-polylactic acid coupled prodrug 3 ( image 3 )
[0061] Add mPLA(2600)-SA (591 mg, 0.20 mmol) and SN38 (79 mg, 0.20 mmol) into a 100 mL round bottom flask, dissolve in 15 mL of anhydrous dichloromethane, and add EDC (47 mg, 0.30 mmol). Stir at 50°C for 4 hours, remove the reaction solvent, and wash with 5% citric acid, saturated sodium bicarbonate, and saturated brine, respectively. The organic phase was dried over anhydrous sodium sulfate, filtered, the filtrate was collected and the solvent was removed under reduced pressure. Product 3 (320 mg, 48%) was obtained after separation and purification by column chromatography (DCM:MeOH=120:1).
[0062] Prodrug 3 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0063] 1 HNMR (400MHz, CDCl 3 ):δ1.02-1.06(t,3H),1.38-1.42(t,3H),1.53-1.61(m,108H),1.86-1.92(m,2H),2.89-2.92(m,2H),2.98 -3.03(m,2H),3.13-3.19(q,2H),3.38(s,3H),3.53-3.56(q,2H),3.63-3.65(t,2H),3.68-3.70(t,2H) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com