Aromatic secondary primary amine containing triaryl-s-triazine structure and ether bond and preparation method thereof
A dibasic primary amine, s-triazine technology, applied in the field of compound synthesis, can solve the problems of high flexibility of ether bonds, decrease of glass transition temperature and thermal decomposition temperature of polymers, etc., to achieve good solubility and thermoplasticity, and reduce dielectric loss. and water absorption, the effect of improving heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The preparation method of the above-mentioned aromatic dibasic primary amine containing triaryl-s-triazine structure and ether bond specifically comprises the following steps in sequence:
[0048] Step 1, add p-aminophenol compounds such as the structure shown in formula (III) (i.e. amine source) and alkali catalyst (the molar ratio of the two is 1.0: (0.1-5.0)), solvent, and dehydrating agent are sequentially added to the A water separator, a stirrer, a thermometer and an inert atmosphere (preferably nitrogen or argon) are introduced into the reactor for reflux reaction for 0.5-8.0 hours, the reaction time is preferably 2 hours, and the dehydrating agent is separated. On the one hand, due to the reaction of p-aminophenol compounds with strong bases (such as sodium hydride, potassium hydride, etc.) to generate water, they will compete with phenoxy salts, resulting in increased side reactions; on the other hand, due to the use of amine sources, bases and solvents Contain...
Embodiment 1
[0058] (1) 0.2mol of 4-aminophenol (that is, R in formula (Ⅲ) 4 and R 5 All hydrogen), 0.14mol of potassium carbonate, 200mL of N-methyl-2-pyrrolidone (NMP) and 200mL of toluene were added into the reaction flask, nitrogen gas was passed, and the toluene was evaporated after reflux with water for 2 hours.
[0059] (2) Cool the reaction flask to room temperature, add 0.1mol 2,4-bis(4-fluorophenyl)-6-phenyl-1,3,5 triazine (BFPT, that is, R in formula (II) 1 and R 2 , R 3 Both are hydrogen), heated to 160°C for 12h, and cooled to room temperature.
[0060] (3) Precipitate the product in cold water, filter (recover the mother liquor, and recycle), collect the solid, and repeatedly rinse with warm water several times to remove inorganic salts and solvents.
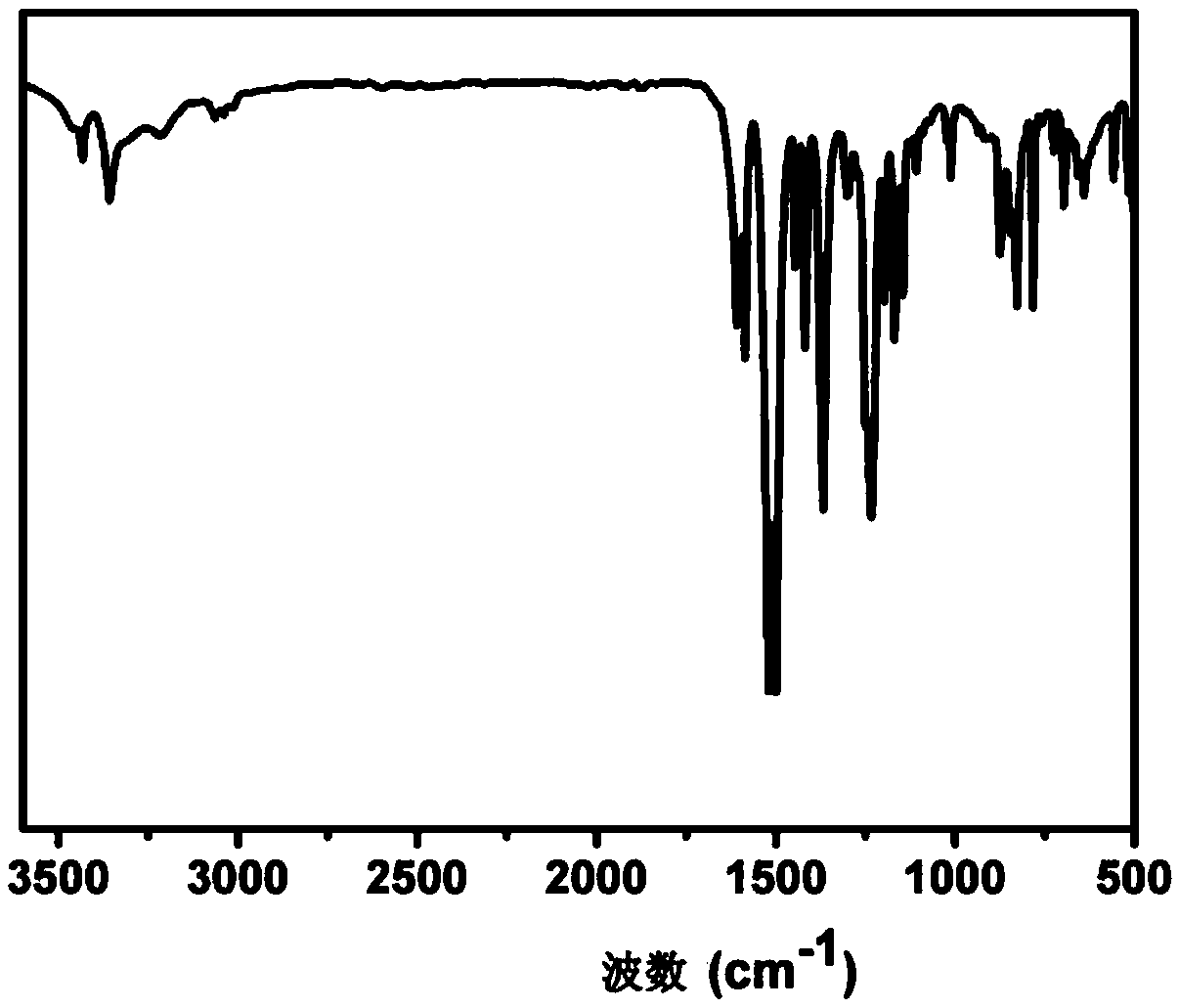
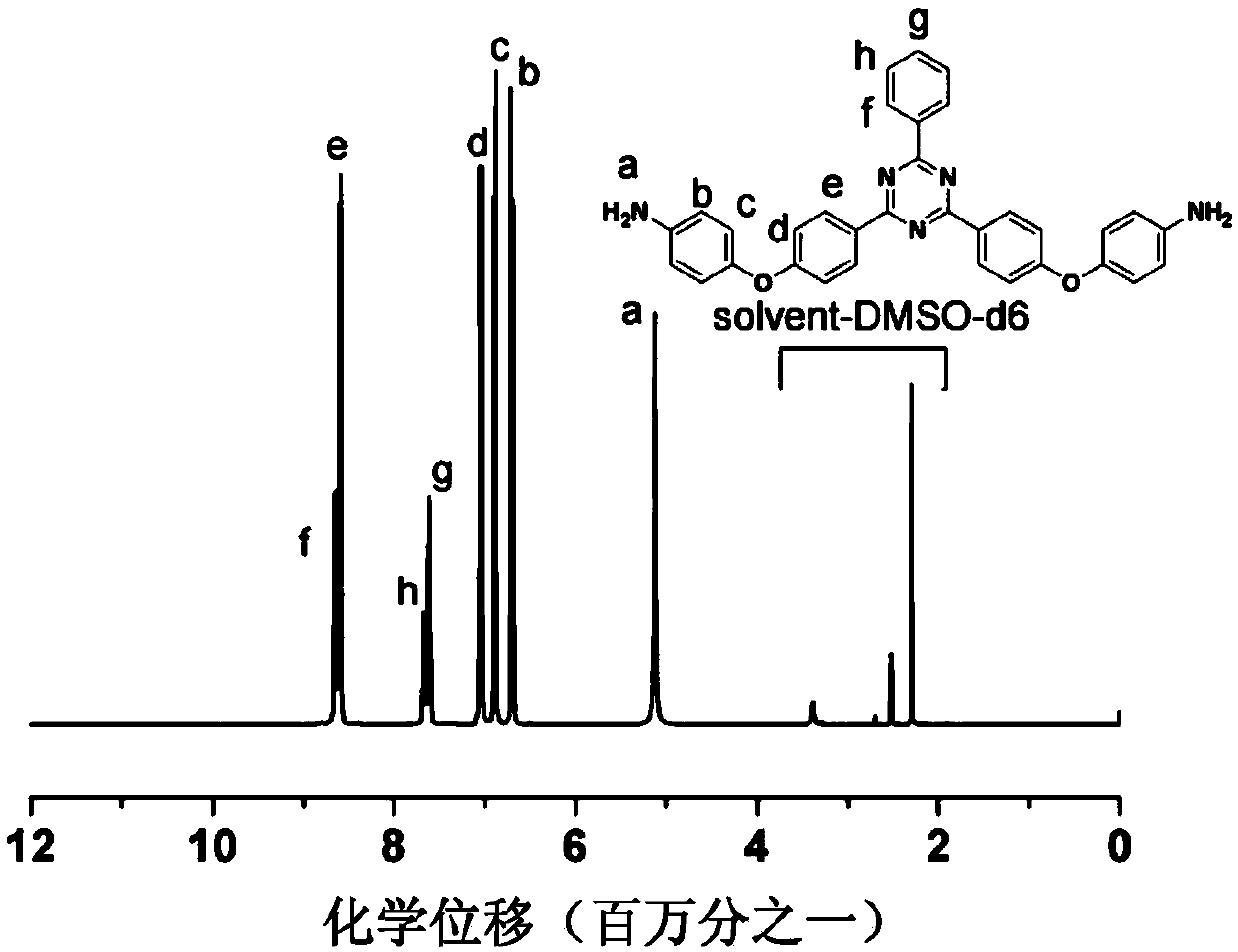
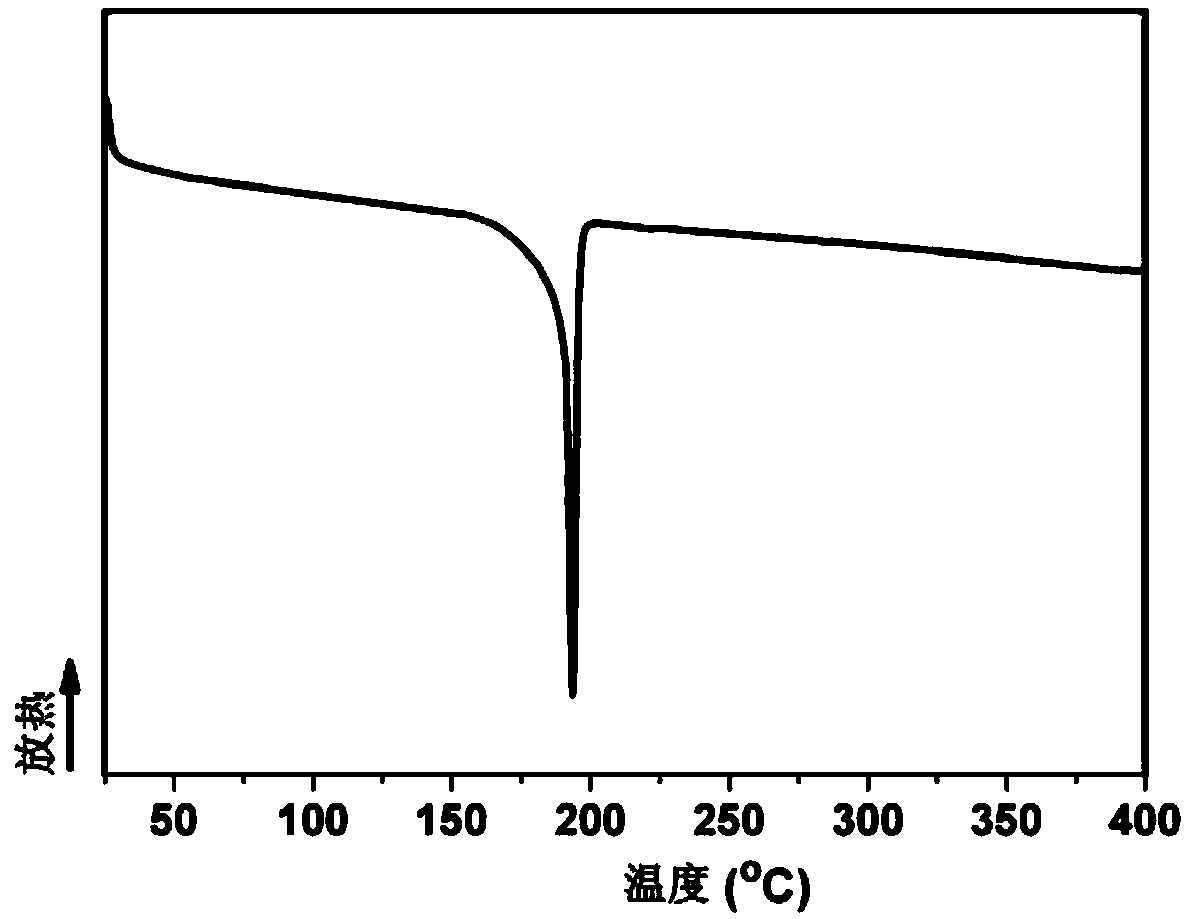
[0061] (4) Collect the filter cake and dry it under vacuum to obtain 49.6g of tan 2,4-two 4-(4-aminophenoxy)phenyl-6-phenyl-1,3,5-triazine product (the structure is as follows: shown), the molar yield was 94.6%, and the pu...
Embodiment 2
[0079] (1) Add 0.2 mol of 3,5-dimethyl-4-aminophenol, 0.2 mol of sodium hydroxide, 100 ml of N-methylpyrrolidone (NMP) and 50 ml of toluene into the reaction flask, ventilate nitrogen, and bring water under reflux After reacting for 2h, toluene was distilled off.
[0080] (2) Cool the reaction flask to room temperature, add 0.1mol 2,4-bis(3,5-dimethyl-4-fluorophenyl)-6-phenyl-1,3,5 triazine, and heat up to React at 160°C for 12h, then cool to room temperature.
[0081] (3) Precipitate the product in cold water, filter (recover the mother liquor, and recycle), and repeatedly rinse with warm water several times to remove inorganic salts and solvents.
[0082] (4) Collect the filter cake and dry it in vacuum to obtain tan 2,4-bis[3,5-dimethyl-4-(3,5-dimethyl-4-aminophenoxy)phenyl]-6- The phenyl-1,3,5-triazine product (structure shown in the following formula) has a molar yield of 91.5% and a purity of 99%.
[0083]
[0084] R in the structure 1 ~R 2 , R 4 ~R 5 Both are ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com