Novel medicine composition capable of stimulating collagen secretion and uses thereof
A composition and drug technology, applied in the field of biomedicine, to achieve the effects of drug safety, promotion of vascularization, and a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] In this example, the inventors screened mesenchymal stem cells from different sources with collagen or hyaluronic acid or calcium hydroxyapatite (microcrystalline porcelain) or polylactic acid or adipose or platelet-rich plasma. The effect of improving cell activity. In this example, the cells to be increased are fibroblasts. Mesenchymal stem cells from different sources include: cord blood, peripheral blood, umbilical cord, placenta, Derived from amniotic membrane, derived from bone marrow, derived from periosteum, derived from fat, derived from teeth, etc. The inventor found that compared with mesenchymal stem cells from other sources, mesenchymal stem cells derived from umbilical cords are medical wastes, with lower cellular immunogenicity and certain versatility. The obtained cells are of high quality and large quantity. , The purity is also higher. Furthermore, in subsequent experiments, the inventors selected a pharmaceutical composition comprising umbilical cord-...
Embodiment 2
[0062] In this example, the inventor observed and identified the morphology and phenotype of the selected umbilical cord mesenchymal stem cells. The results are as follows:
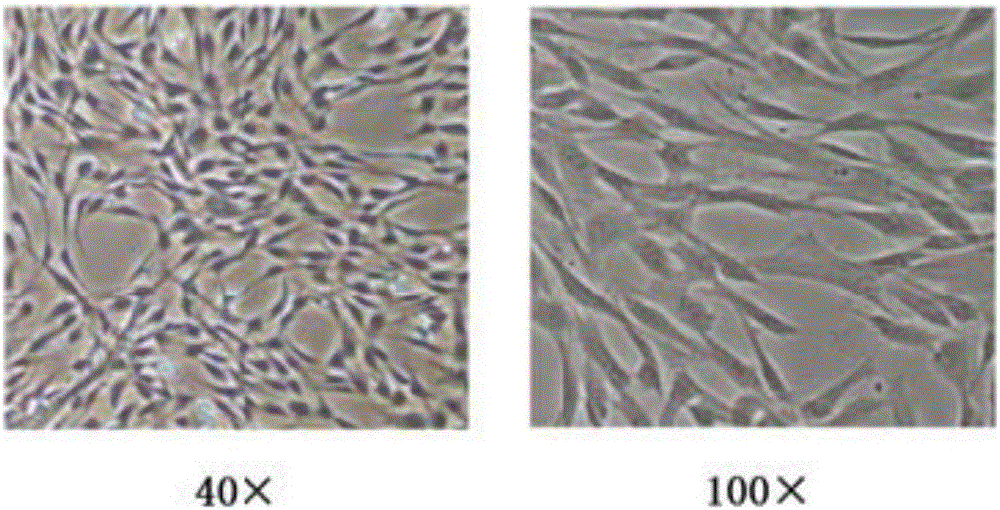
[0063] The morphology of umbilical cord mesenchymal stem cells is that the cells adhere to the wall and grow in the shape of a spindle and sheet. Specific form such as figure 1 Shown. Umbilical cord mesenchymal stem cells are in such figure 1 In the growing state, the umbilical cord mesenchymal stem cells have strong proliferation ability, secrete cytokines including bFGF, VEGF, IGF, etc., and have strong proliferation ability of collagen, and are suitable for selection as the umbilical cord mesenchyme of the pharmaceutical composition of the present invention stem cell.
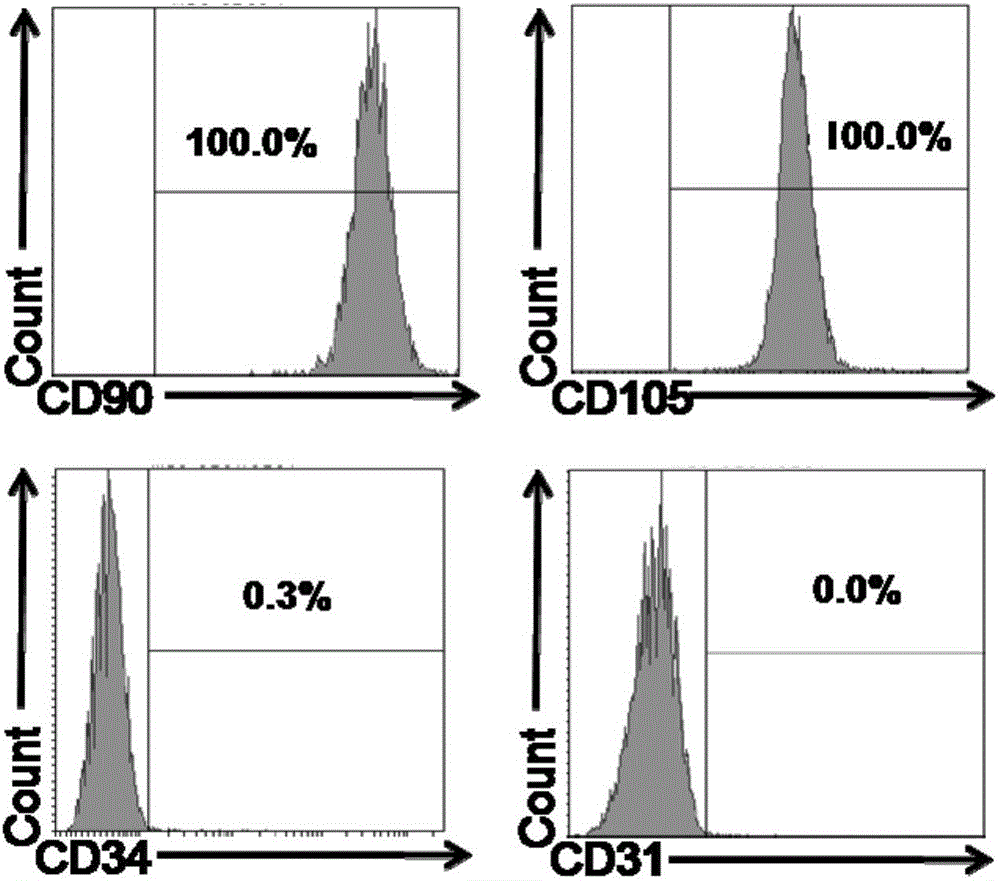
[0064] Flow cytometric identification of the obtained umbilical cord mesenchymal stem cells, the results figure 2 Shown from figure 2 It can be seen from the results that umbilical cord mesenchymal stem cells express CD90 (positive cell ...
Embodiment 3
[0066] In this embodiment, the inventor further pre-stimulated the obtained umbilical cord mesenchymal stem cells with TGF-β1, bFGF or a combination of the two. The pre-stimulated mesenchymal stem cells were injected into the mouse dermis and subcutaneous fat layer. The inventor found that this is more conducive to the secretion of collagen, more effectively activates autologous cells and stimulates the formation of blood vessels, so that more nutrients are transported to the skin of wrinkles. In this embodiment, the specific dosage of 2-20ng / ml TGF-β1, 5-30ng / ml bFGF, or both are added to pretreat umbilical cord mesenchymal stem cells. The pretreatment time is 1-5 days.
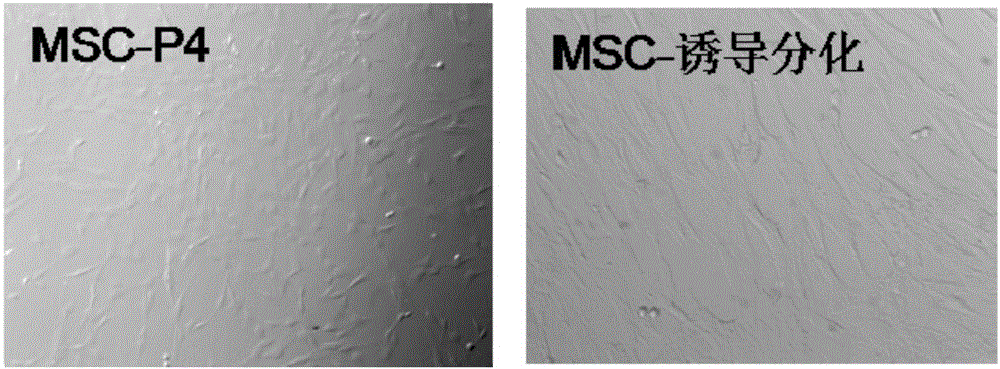
[0067] The inventors found that the umbilical cord mesenchymal stem cells induced to differentiate 3D in the medium supplemented with TGF-β1 and bFGF are as follows image 3 As shown, it can be seen from the results that compared with the normal morphology of mesenchymal stem cells, the induced differentiation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com