Preparation method of Alpha-naphthyl containing diarylketone compound
A technology for naphthyl diaryl ketones and compounds is applied in the field of preparation of compounds containing α-naphthyl diaryl ketones, which can solve the problems of inconsistent concepts, poor regioselectivity, and high reaction temperature, and achieves simple operation, mild conditions, and high yields. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
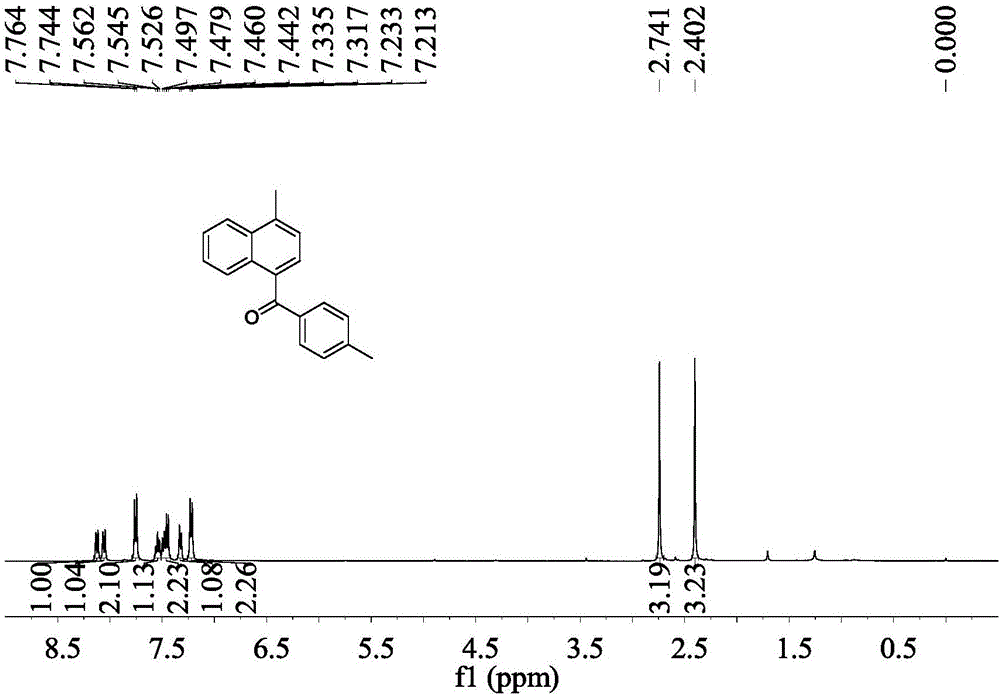
[0042] Embodiment 1: the synthesis of 4-methyl-(4'-methylphenyl)-1-naphthyl ketone
[0043]In a 25mL reactor, sodium tert-butoxide (0.058g, 0.6mmol) and tetrakis(triphenylphosphine)palladium (0.017g, 0.015mmol) were added, and after nitrogen replacement 3 times, 5mL of tetrahydrofuran was added, and 4- Tolylbenzeneacetonitrile (0.079g, 0.6mmol) and 1-chloromethylnaphthalene (0.053g, 0.3mmol) were stirred at 30°C for 12h, and the reaction solution was stirred in air for 10h. Separation by column chromatography (silica gel, 200-300 mesh; developer, petroleum ether: ethyl acetate = 50:1) to obtain 0.064 g of 4-methyl-(4'-methylphenyl)-1-naphthyl ketone , yield 82%.
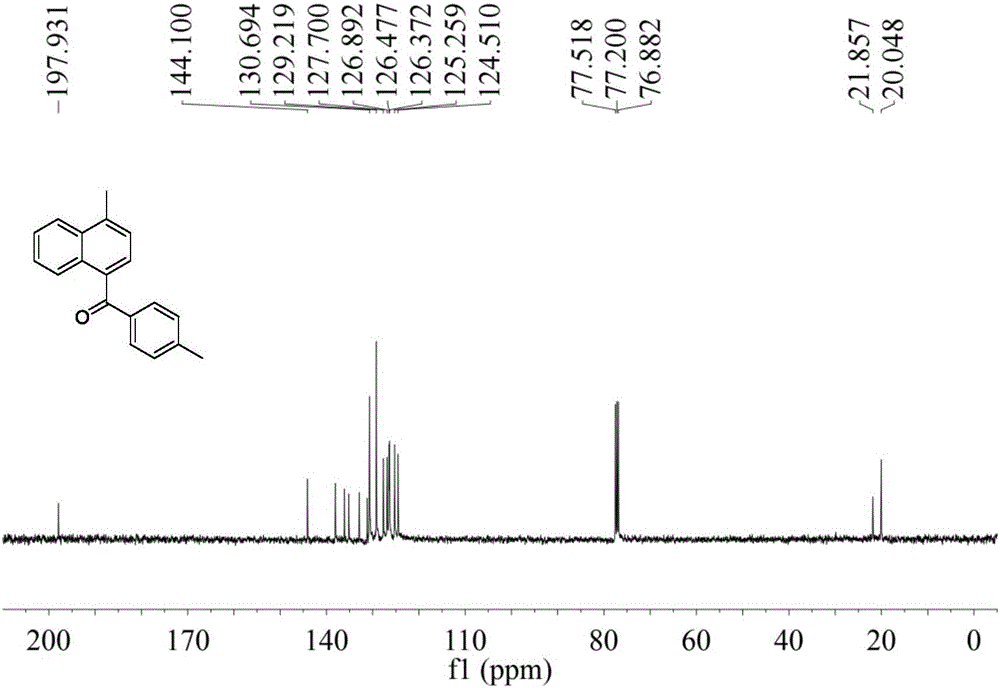
[0044] 4-Methyl-(4′-methylphenyl)-1-naphthylmethanone
[0045] Pale yellow oily liquid; IR(neat)ν2955,2924,2854,1656,1604,1513,1456,1284,1254,1178,973,833,762cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ2.40(s,3H),2.74(s,3H),7.22(d,J=8.0Hz,2H),7.33(d,J=7.2Hz,1H),7.44–7.50(m,2H), 7.54(dd, J=6.8,7.6Hz,1H),7.75(d,J=8.0Hz,2...
Embodiment 2
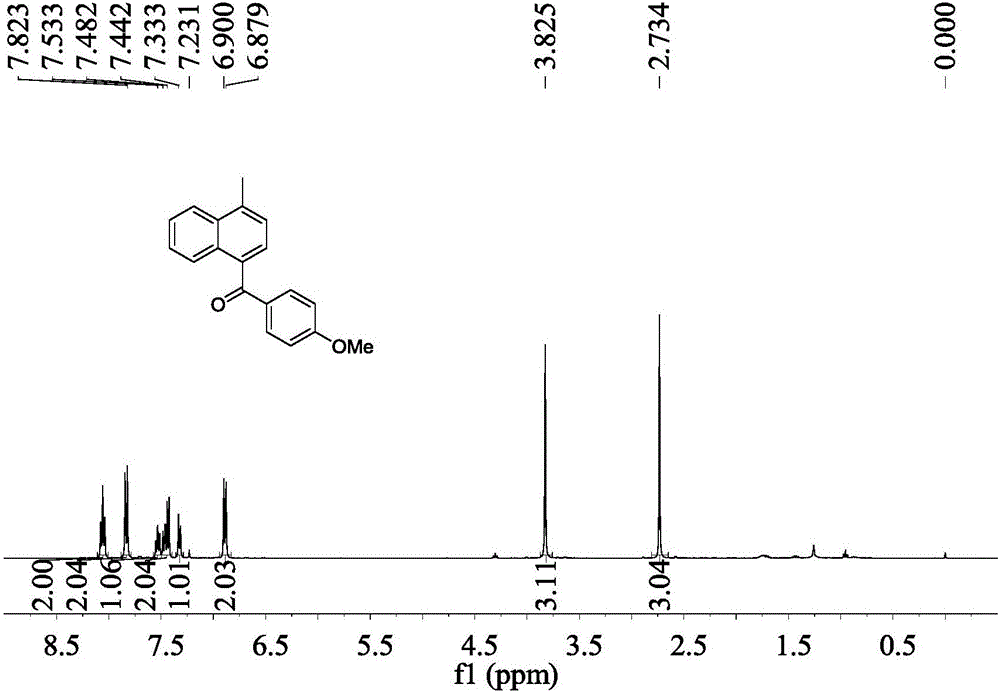
[0046] Embodiment 2: the synthesis of 4-methyl-(4'-methoxyphenyl)-1-naphthyl ketone
[0047] Operation is the same as in Example 1, and 0.070 g of 4-methyl-(4'-methoxyphenyl)-1-naphthyl ketone is obtained by the reaction of 1-chloromethylnaphthalene and 4-methoxyphenylacetonitrile. 84%.
[0048] 4-Methyl-(4′-methoxyphenyl)-1-naphthylmethanone
[0049] Pale yellow solid, mp 77.8-78.3℃; IR(KBr)ν3071,2932,2839,1651,1598,1509,1254,1170,1029,973,878,845,768cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ2.73(s,3H),3.83(s,3H),6.89(d,J=8.4Hz,2H),7.32(d,J=7.2Hz,1H),7.42–7.48(m,2H), 7.53(dd, J=7.2,8.0Hz,1H),7.83(d,J=8.8Hz,2H),8.06(dd,J=7.6,8.0Hz,2H); 13 C NMR (100MHz, CDCl 3 )δ20.0,55.6,113.7,124.5,125.3,126.3,126.5,126.8,127.1,131.1,131.4,132.9,135.5,137.7,163.8,196.9; HRMS(EI,m / z) calcd for C 19 h 16 o 2 :276.1150[M] + ;found: 276.1151.
Embodiment 3
[0050] Example 3: Synthesis of 4-methyl-(4'-bromophenyl)-1-naphthyl ketone
[0051] The operation was the same as in Example 1, and 0.071 g of 4-methyl-(4'-bromophenyl)-1-naphthyl ketone was obtained by reacting 1-chloromethylnaphthalene with 4-bromophenylacetonitrile, with a yield of 73%.
[0052] 4-Methyl-(4′-bromophenyl)-1-naphthylmethanone
[0053] Yellow solid, mp 75.7-76.0℃; IR(KBr)ν3067,2923,2860,1656,1583,1514,1396,1283,1254,1070,1010,974,875,841,763cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ2.76(s,3H),7.34(d,J=7.2Hz,1H),7.45(d,J=7.2Hz,1H),7.50–7.59(m,4H),7.71(d,J=8.0 Hz, 2H), 8.08(d, J=8.0Hz, 1H), 8.15(d, J=8.4Hz, 1H); 13 C NMR (100MHz, CDCl 3 )δ20.1, 124.6, 125.2, 126.3, 126.6, 127.2, 128.3, 128.4, 131.1, 131.8, 132.0, 133.0, 134.2, 137.5, 138.4, 138.9, 197.0; HRMS (EI, m / z) calcd for C 18 h 13 OBr:324.0150[M] + ;found: 324.0148.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com