Furazolidone-metabolite-resistant monoclonal antibody and application thereof
A technology of monoclonal antibody and furazolidone, which is applied in the analysis of veterinary drug residues and the field of immunology, can solve the problems of high experimental equipment and technical requirements, cumbersome operation, and is not suitable for rapid detection of large batches of samples, and achieves strong controllability and repeatability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 Preparation of furazolidone metabolite antigen of the present invention
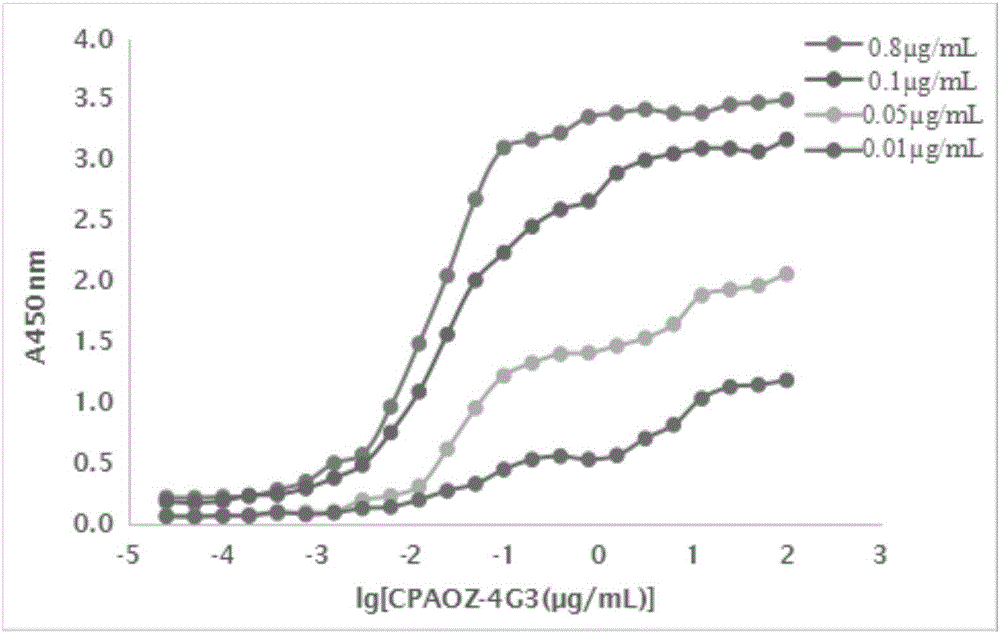
[0041] (1) Derivatization of furazolidone metabolites: take 0.25g AOZ, dissolve it with about 2mL of methanol at room temperature; take 0.15g of 3-carboxybenzaldehyde, dissolve it with 2mL of methanol, slowly drop into the above In the AOZ solution, stir and react at room temperature for 5 hours, filter, and dry the filter residue to obtain the furazolidone metabolite derivative CPAOZ;
[0042] (2) Synthesis of immunogen: Weigh 50 mg of bovine serum albumin (BSA) and dissolve it in 1 mL of 0.1 mol / L PBS with a pH value of 7.4, add 1 mL of N,N-dimethylamide (DMF), stir to dissolve ; Dissolve 8 mg of CPAOZ obtained in step (a) in 1 mL of DMF solution, add 14 mg of dicyclohexylimide (DCC) and 6 mg of N-hydroxysuccinimide (NHS) under stirring, and stir overnight at 4° C., 8000r Centrifuge at 1 / min for 15 minutes, collect the supernatant and put it into a dialysis bag, dialyze with 0.01mo...
Embodiment 2
[0044] Example 2 Preparation of monoclonal antibody to furazolidone metabolites according to the present invention
[0045] (1) Animal immunization: immunize 6-8 week-old female Blab / c mice with the immunogen whose carrier protein is bovine serum albumin, and immunize once every 2 weeks. Determine the titer and inhibition rate, and select the mouse with the best result to prepare for fusion;
[0046] Table 1 Immunization flow chart
[0047]
[0048]
[0049] (2) Cell fusion: the fused mice were bleed from the eyeballs, and the serum was used as a positive control. After the neck was killed, the spleen was taken out under aseptic conditions to prepare spleen cells, which were fused with SP2 / 0 cells by PEG at a ratio of 5:1. The fused cell suspension was added to a 96-well plate covered with feeder cells, and cultured in an incubator at 37°C and 5% CO2;
[0050] (3) Screening of positive hybridoma cell lines: the fused cells were checked for contamination the next day, a...
Embodiment 3
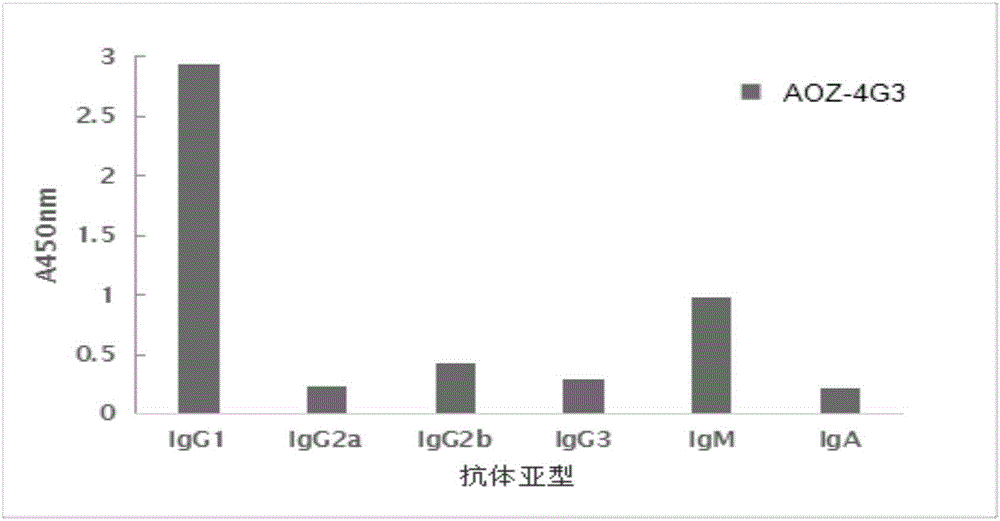
[0052] Example 3 Characteristic Identification of Furazolidone Metabolite Monoclonal Antibody According to the Present Invention
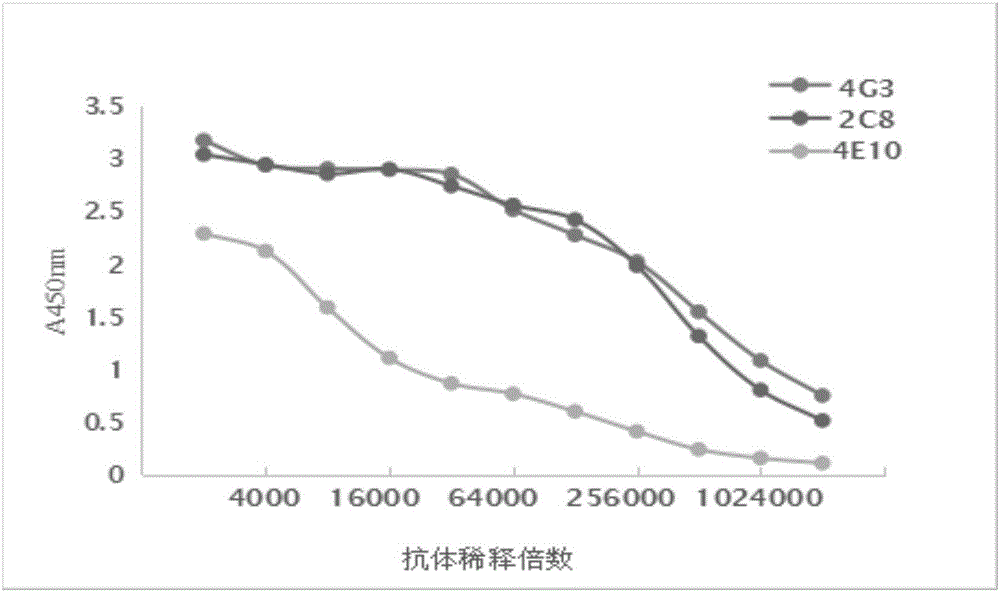
[0053] (1) Potency determination
[0054] The indirect ELISA method is used, and the specific steps are as follows: Coating: Dilute the coating material with carbonate buffer solution to a concentration of 2 μg / mL, 100 μL / well in a 96-well microtiter plate, overnight at 4°C; washing: return the coated plate to room temperature , pour off the coating solution, add 300 μL of washing solution to each well, let stand for 1 min each time, wash 3 times, and pat dry for the last time; sealing: add 200 μL of washing solution containing 10% calf serum to each well, 37°C for 1 hour; Pour off the blocking solution, wash 3 times, and pat dry; add primary antibody: use the washing solution to dilute the monoclonal antibody at a ratio of 1:2000, add 100 μL to each well, and set a blank control well (PBS) and a negative control (negative control) Serum), placed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| reaction rate constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com