A kind of mn4+ doped sodium hydrogen fluoride red light material and preparation method thereof
A technology of sodium hydrogen fluoride and red light, which is applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problems of expensive raw materials and complicated synthesis methods, and achieve the effect of simple raw materials, pure red light and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
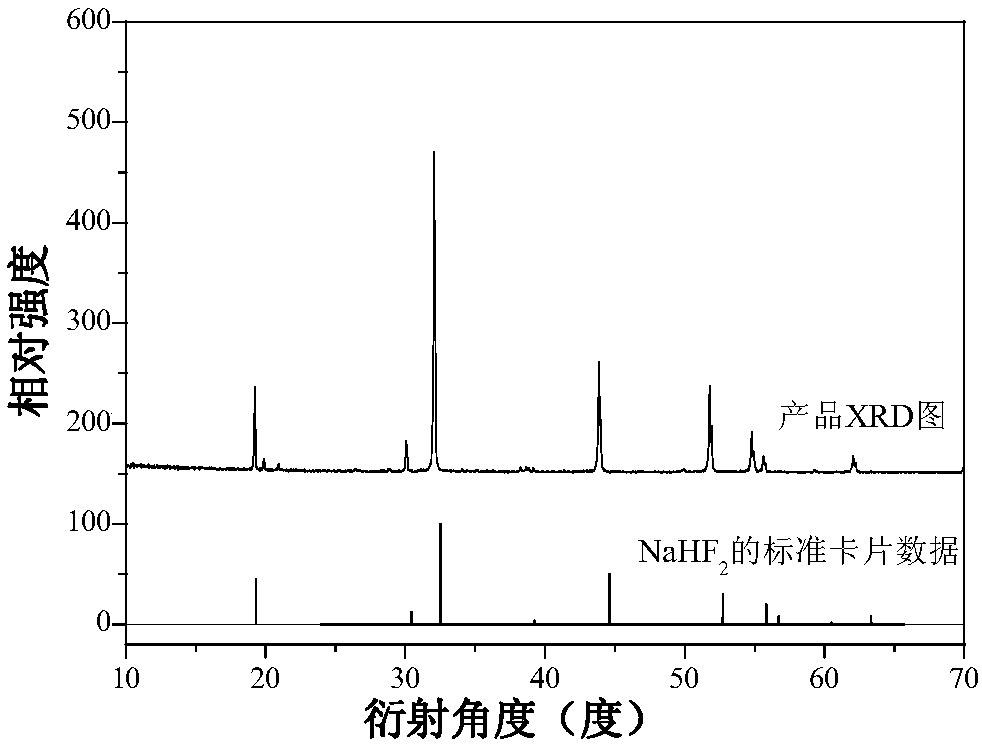
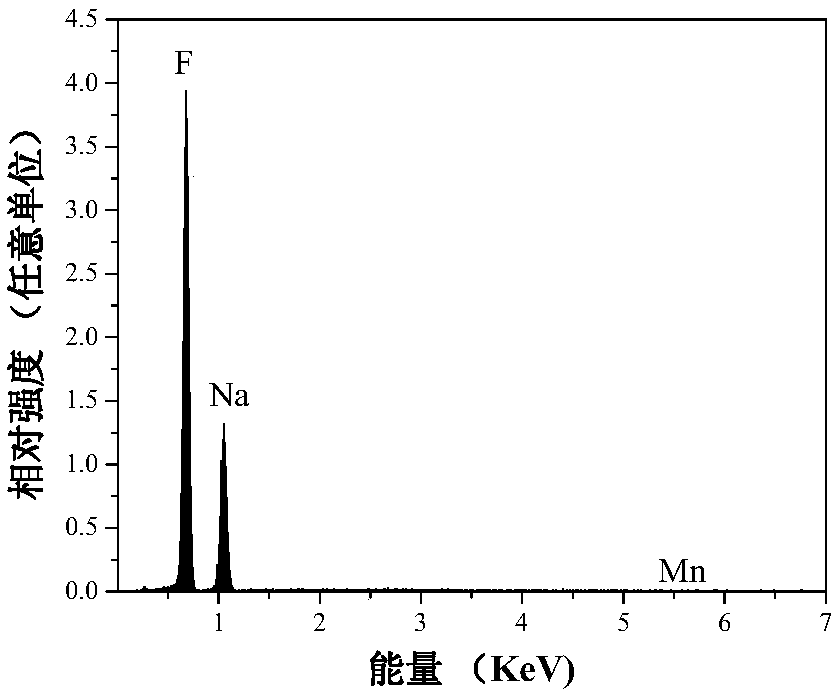
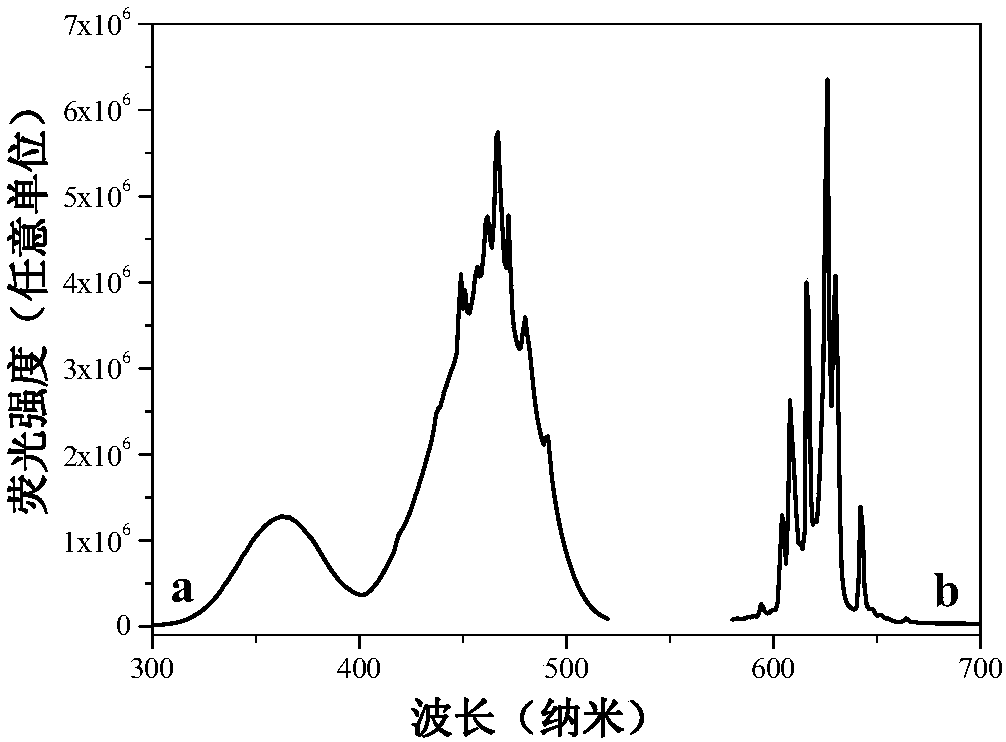
[0025] In a plastic container, mix 0.1235g (5×10 ‐4 mol)K 2 MnF 6 Dissolve solid material in 20mL HF (concentration: wt40%), then add 2.1g (0.05mol) NaF as raw material, add deionized water to make the total volume 40mL, stir and react at room temperature for 1.5 hours, filter with suction, and dry naturally at room temperature , to obtain a white powder. The product glows red under a UV light. Its XRD (Bruker D8Advance X-ray diffractometer detection) such as figure 1 As shown, XRD shows that the product is pure NaHF 2 phase, slightly doped with Mn 4+ Did not significantly affect the phase. Such as figure 2 As shown, the energy spectrum analysis is measured on the Nova NanoSEM 200. Under the action of the electron beam, the energy spectrum analysis shows elements: Na, F and Mn, and H cannot be displayed because the mass is too small. It can be seen that the obtained product composition is NaHF 2 :Mn 4+ . Such as image 3 As shown, using Fluoromax‐4 fluorescence spe...
Embodiment 2
[0027] In a plastic container, mix 0.0988g (4×10 ‐4 mol)K 2 MnF 6 Dissolve the solid material in 30mL HF (concentration: wt40%), then add 0.42g (0.01mol) NaF as raw material, add deionized water to make the total volume 40mL, stir and react at room temperature for 0.5 hours, filter with suction, and dry naturally at room temperature , to obtain a white powder. The product glows red under a UV light. The XRD figure, scanning electron microscope picture and fluorescence spectrum of the white powder material and figure 1 ‐3 are basically the same.
Embodiment 3
[0029] In a plastic container, mix 0.1482g (6×10 ‐4 mol)K 2 MnF 6 Dissolve solid material in 15mL HF (concentration: wt40%), then add 3.36g (0.08mol) NaF as raw material, add deionized water to make the total volume 40mL, stir and react at room temperature for 1 hour, filter with suction, and dry naturally at room temperature , to obtain a white powder. The product glows red under a UV light. The XRD figure, scanning electron microscope picture and fluorescence spectrum of the white powder material and figure 1 ‐3 are basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com