Preparation method of everolimus solid dispersion
A solid dispersion, everolimus technology, applied in antitumor drugs, powder delivery, drug combination and other directions, can solve the problems of difficult material handling, low drying efficiency, etc., to improve drying efficiency, avoid oxidative degradation, and surface crispy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
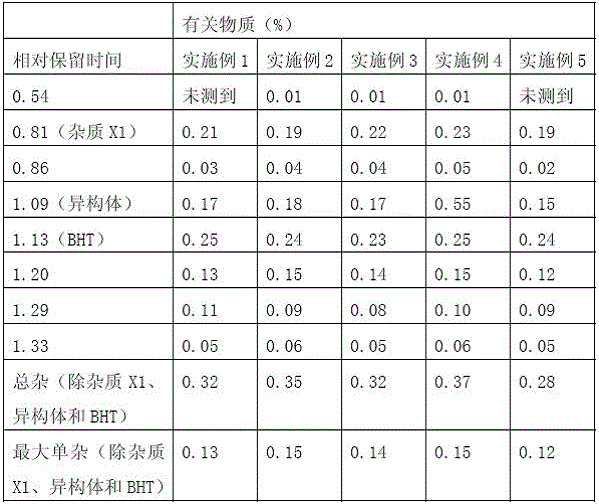
Examples
Embodiment 1)
[0025] The preparation method of the everolimus solid dispersion of the present embodiment has the following steps:
[0026] ① Mix 1 g of everolimus, 8.98 g of hydroxypropyl methylcellulose (E3 PREMIUM LV1 from Dow) and 0.02 g of 2,6-di-tert-butyl-4-methylphenol and add to 100 mL of ethanol and 100 mL of acetone, and stir to mix the materials evenly.
[0027] ② Spread the material obtained in step ① evenly on the flat rotating sample tray of the microwave vacuum dryer, turn on the vacuum pump, control the vacuum degree to be ≤-0.085MPa, pre-equilibrate for 2 minutes, set the microwave power to 518W, turn on the microwave function, and wait for the material After the temperature rose to 40°C, microwave vacuum drying was completed.
[0028] Discharge, and then lay the material flat in a vacuum drying oven at 50°C and a vacuum degree of ≤-0.085MPa, and continue to remove the organic solvent for 24h.
[0029] Discharge and pulverize to obtain solid dispersion powder.
Embodiment 2)
[0031] The preparation method of the everolimus solid dispersion of the present embodiment has the following steps:
[0032] ①Mix 10g of everolimus and 30g of hydroxypropyl methylcellulose (E3 PHARM from Ashland), add 1000mL of ethanol, and stir to mix the materials evenly.
[0033] ② Spread the material obtained in step ① evenly in the sample tray of the three-dimensional rotating support of the microwave vacuum dryer, turn on the vacuum pump, control the vacuum degree to be ≤-0.090MPa, pre-equilibrate for 10 minutes, set the microwave power to 550W, turn on the microwave function, wait for After the temperature of the material rose to 50°C, microwave vacuum drying was completed.
[0034] Discharge and pulverize to obtain solid dispersion powder.
Embodiment 3)
[0036] The preparation method of the everolimus solid dispersion of the present embodiment has the following steps:
[0037] ① Mix 1 g of everolimus, 20 g of hydroxypropyl methylcellulose (630 from Shin-Etsu), 3.9 g of lactose monohydrate and 0.1 g of dl-α-tocopherol acetate, then add 300 mL of methanol and 200 mL of methanol of acetone, and stir to mix the materials evenly.
[0038] ② The material obtained in step ① is evenly spread on the material belt of the belt-type continuous production microwave vacuum continuous dryer through the feeding device, and the vacuum degree is controlled to be ≤-0.080MPa, and the conveying speed and microwave power are adjusted. The material device collects the material.
[0039] Discharge and pulverize to obtain solid dispersion powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com