Cationic polymerization monomers, and synthesis and application thereof
A technology of compound and carbon atom, which is applied in the field of cationic polymerization monomer and its synthesis and application, can solve the problems of severe polymerization inhibition, inconvenient operation, volume shrinkage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Therefore, in a variant of the preparation method of the present invention, there is provided a method for the preparation of a compound of formula (I) or (II), comprising
[0037] 1) reacting the compound of formula (I') or (II') with a hydride base selected from alkali metal hydrides and alkaline earth metal hydrides under anhydrous conditions, and then reacting with the compound of formula (IV) under anhydrous conditions ,
[0038]
[0039] Obtain formula (I ") or (II ") compound respectively,
[0040]
[0041]
[0042] as well as
[0043] 2) make the fluorine-containing alcohol of formula OH-Y react with the hydride base selected from alkali metal hydride and alkaline earth metal hydride under anhydrous conditions, and then react with formula (I ") or (II) under anhydrous conditions ") compound reaction, obtain formula (I) or (II) compound respectively,
[0044] where the variable R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , Y, X, m and n are as defi...
Embodiment 1
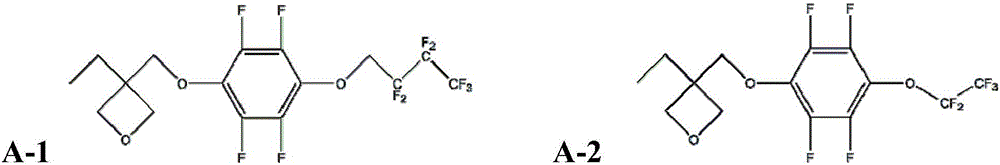
[0080] Embodiment 1: the synthesis of monomer A-1
[0081] Method 1 (method including step a) and step b): under nitrogen atmosphere, add 2.232g (0.012mol) 2,2,3,3,4,4,4-heptafluorobutanol and 70mL anhydrous toluene Stir evenly in a 250mL three-necked bottle. Under an ice-water bath, 0.52 g (0.013 mol) of potassium hydride was added into the three-necked flask three times at intervals of 2 minutes each time and stirred rapidly. Then, under an ice-water bath, slowly add a solution of 1.86g (0.01mol) hexafluorobenzene in 100ml of anhydrous toluene dropwise to the three-necked flask at a rate of about 4 drops / s, and drop it in about 20 minutes, then remove the ice-water bath , stirred at room temperature for 22h. After the reaction is completed, add 20ml of water to quench the reaction, the product is washed with water (3*50ml), extracted with petroleum ether (3*50ml), the extract is dried with magnesium sulfate, the petroleum ether is removed by rotary evaporation, and the int...
Embodiment 2
[0085] Embodiment 2: the synthesis of monomer A-2
[0086] Repeat the first method of Example 1, the difference is: 2,2,3,3,4,4,4-heptafluorobutanol is replaced by perfluoroethanol, the solvent is replaced by anhydrous DMF, and potassium hydride is replaced by hydrogenation Calcium, the stirring time at room temperature in the first step was changed to 24h, and the stirring time at room temperature in the second step was changed to 20h. The intermediate product 2g was obtained with a yield of 80%; the final product A-2 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com