Levodopa crystalline powder and preparation method thereof
A technology of crystalline powder and manufacturing method, applied in the directions of organic chemistry method, chemical instrument and method, cyanide reaction preparation, etc., can solve the problems of rare levodopa, long reaction time, many by-products, etc., to avoid appearance Effects of browning, improving moisture uniformity, and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
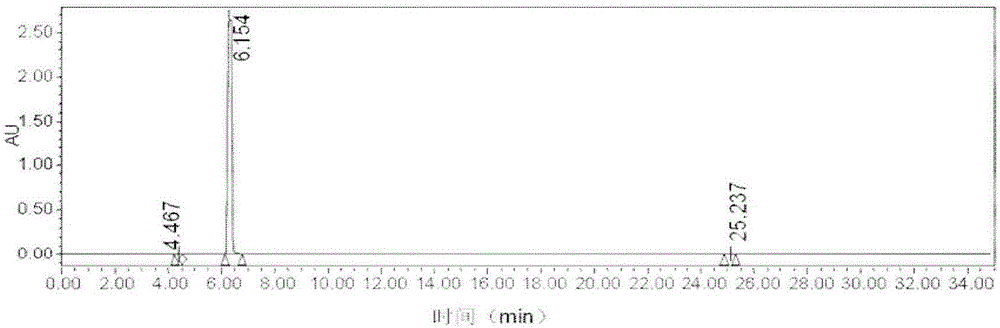
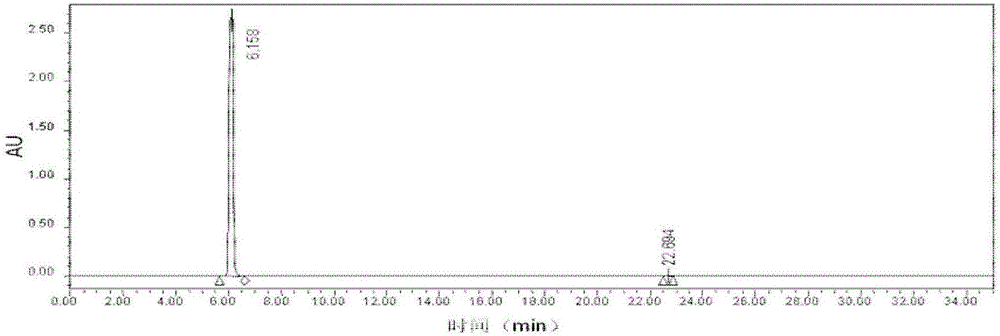
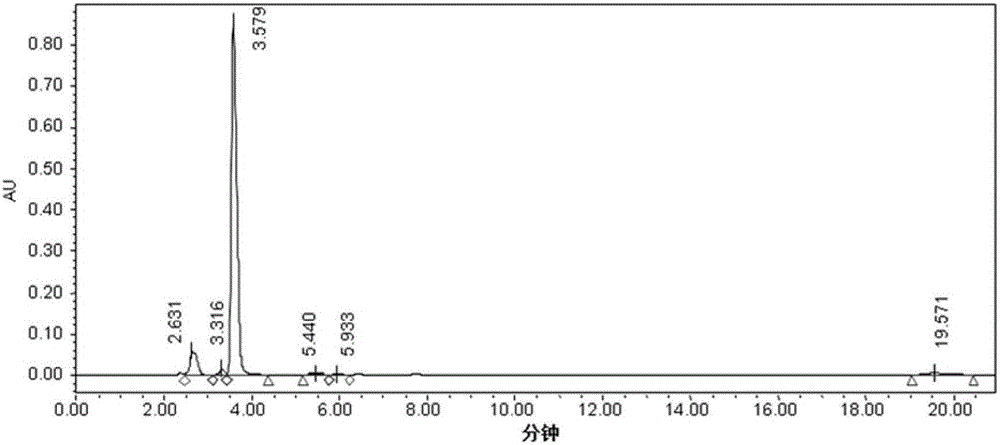
Image
Examples
Embodiment 1
[0032] Embodiment 1 tyrosine phenol lyase biocatalytic process
[0033]Put initial pyruvate into the water, control the pH at 7.0-9.0 with concentrated ammonia water; then add antioxidants, chelating agents, ammonium salts, pyridoxal 5-phosphate in sequence, and control the pH at 7.0-9.0 with concentrated ammonia water again, add The enzyme solution of recombinant tyrosine phenol lyase with a concentration of 80-200g / L is passed through nitrogen, the temperature is adjusted to the reaction temperature, the pH is adjusted to 7.0-9.0 with ammonia water, and catechol is added to start the reaction; the initial basic substrate o The molar ratio of hydroquinone to pyruvic acid and its sodium salt is 1:1-1.5. Afterwards, add the substrates catechol and pyruvate (or ammonium pyruvate, only pyruvate unless otherwise specified) at 2 g / L every 10 minutes, and the final concentration of catechol is 50-70 g / L (calculated based on the initial volume), the final concentration of pyruvic a...
Embodiment 2650
[0038] Example 2 650L conversion system
[0039] Take the levodopa conversion solution obtained in Example 1, add concentrated hydrochloric acid to adjust the pH to 5.1, and obtain a levodopa mixed solution with a total volume of 650 L and a concentration of 110 g / L. The temperature is reduced to 6 ° C. After standing for 4 hours, Use a centrifuge to filter and rinse with a small amount of drinking water. The crude product of levodopa can be collected if no obvious liquid flows out from the material outlet of the centrifuge. The total weight of the crude levodopa collected was 97.5kg, and the levodopa content calculated as dry product was 67.5kg, and the yield of levodopa obtained by centrifuging the crude product was 94.4%.
[0040] Put 97.5kg of crude levodopa obtained by centrifugation into an enamel reaction kettle, add 570L of drinking water, and control the temperature at 30-35°C. Start stirring, add concentrated hydrochloric acid, adjust the pH to 0.97, the amount of c...
Embodiment 36500
[0048] Example 3 6500L conversion system
[0049] Take the levodopa conversion solution obtained in Example 1, add concentrated sulfuric acid with a mass volume ratio of 50% to adjust the pH to 5.4, obtain a total volume of 6500L, and a concentration of levodopa mixed solution of 104g / L, and the temperature drops to 9°C After standing still for 4 hours, press filter with a plate filter, and top wash with a small amount of purified water, and the crude product of levodopa can be collected without obvious liquid flowing out at the outlet of the clear liquid. The total weight of the crude levodopa collected was 858kg, the levodopa content calculated as dry product was 643.6kg, and the yield of levodopa obtained by the plate filter was 95.2%.
[0050] Put 858kg of levodopa crude product obtained by the plate filter into the enamel reaction kettle, add 5700L of drinking water, and control the temperature at 30-35°C. Start stirring, add concentrated hydrochloric acid, adjust the pH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com