AIDS Immunotherapy Adsorber
A technology of immunotherapy and adsorber, which is applied in the field of AIDS immunotherapy adsorber, which can solve the problems of rapid virus mutation, inability to prevent virus rebound, failure, etc., and achieve the effect of high concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
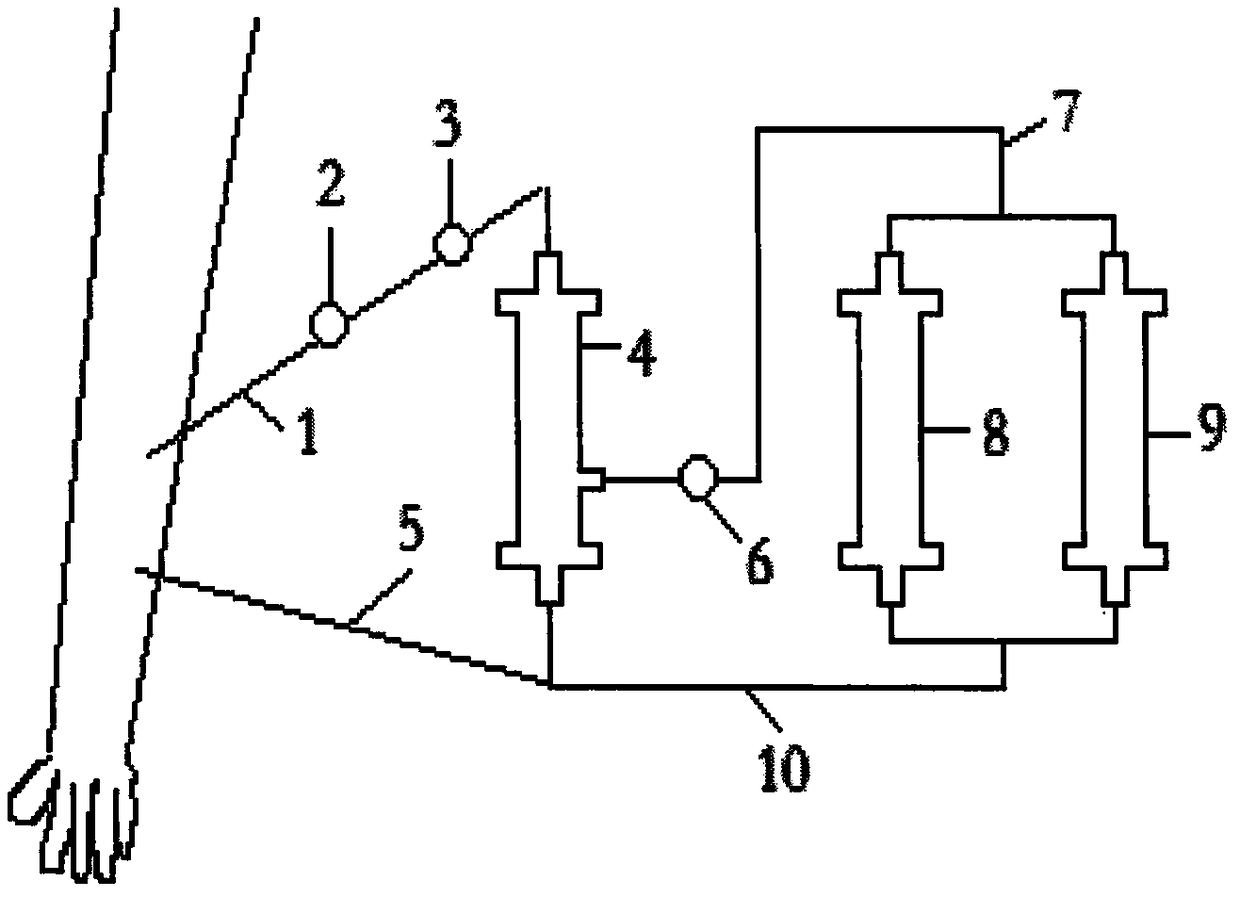
[0012] figure 1 It is an application schematic diagram of the AIDS immunotherapy absorber proposed according to the present invention.
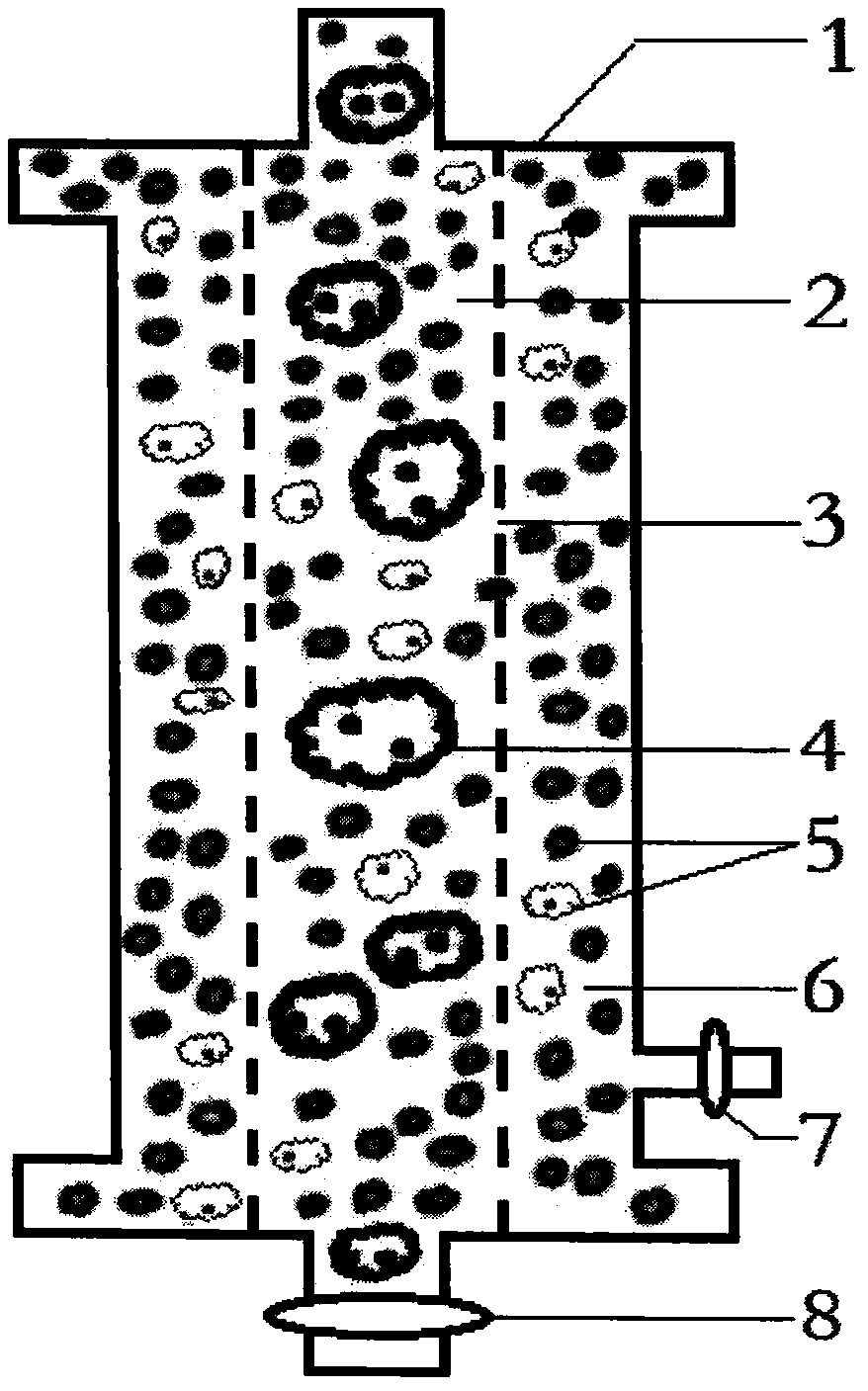
[0013] figure 2 It is the internal structure diagram of the plasma separator proposed according to the present invention.
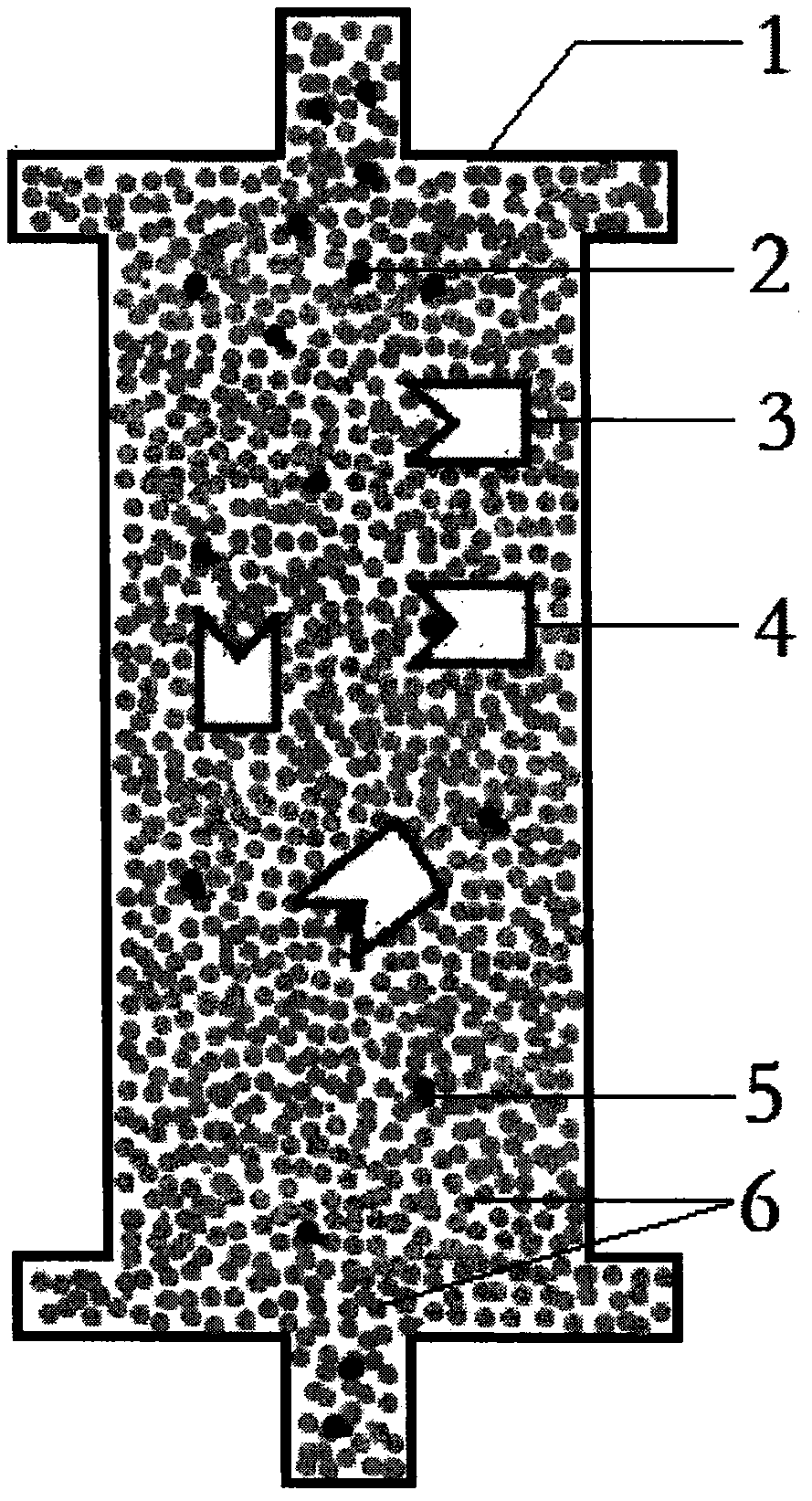
[0014] image 3 It is the internal structure diagram of the AIDS immunotherapy absorber proposed according to the present invention
[0015] figure 1 Among them, one end of the arterial blood line tube (1) is connected with the arterial blood vessel, and the other end is connected with the plasma separator (4) through the heparin pump (2) and the blood pump (3), and the plasma separator (4) is connected through the plasma pump (6) ) and circulation pipeline (7) are connected with two parallel adsorbers (8) and adsorber (9), and then connected with circulation pipeline (10) and venous pipeline (5) in turn, and venous pipeline (5) The other end is connected to the vein.
[0016] figure 2 Among them, 1 is the plasm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com