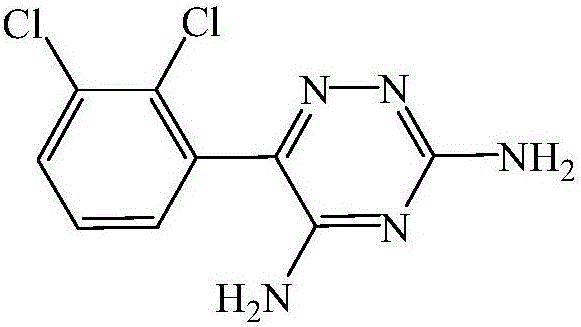
Improved synthesis process for lamotrigine
A synthesis process and lamotrigine technology are applied in the field of improved lamotrigine synthesis technology, which can solve problems such as affecting the yield of product lamotrigine, and achieve the effect of improving quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A kind of improved lamotrigine synthetic technique, concrete implementation steps are as follows:
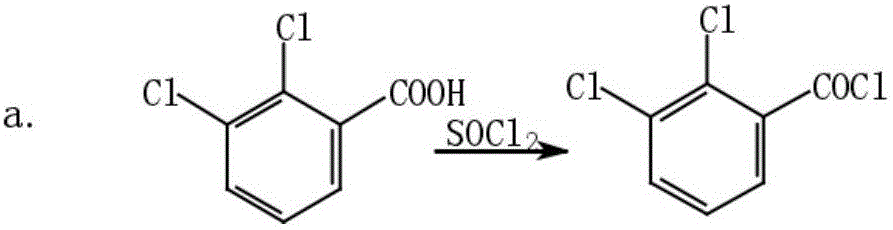
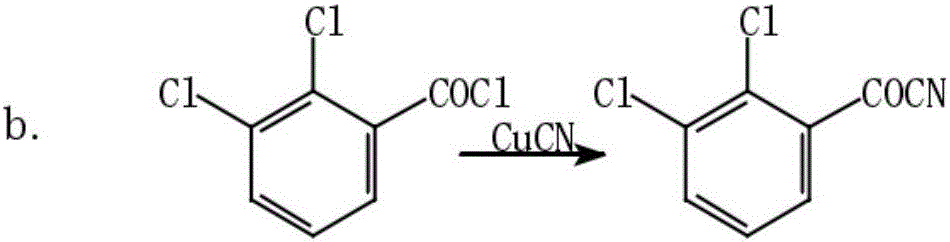
[0031] (1) Synthesis of 2,3-dichlorobenzoyl cyanide: Add 50g of 2,3-dichlorobenzoic acid and 50g of thionyl chloride into a 500ml four-necked bottle, react for 6h to the end, and distill the chlorinated solution under reduced pressure sulfoxide, then add 30g of cuprous cyanide, carefully raise the temperature to 160°C, react for 6h to the end of the reaction, filter out the solid, and get 2,3-dichlorobenzoyl cyanide solution;
[0032] (2) Condensate preparation: add 40g of aminoguanidine carbonate and 200ml of methanol (zeotropic agent) into the reactor, stir and add 30g of concentrated sulfuric acid dropwise, after the addition is completed, the azeotropic agent is evaporated, and water is taken out, and suction filtered , the solid was put into the reaction bottle, stirred at 80°C and vacuum-dried. Add the 2,3-dichlorobenzoyl cyanide solution obtained in step (1), rais...
Embodiment 2
[0041] A kind of improved lamotrigine synthetic technique, concrete implementation steps are as follows:
[0042] (1) Synthesis of 2,3-dichlorobenzoyl cyanide: add 500g of 2,3-dichlorobenzoic acid and 500g of thionyl chloride to the reactor, react for 6h to the end, and distill the thionyl chloride under reduced pressure , then add 30g of cuprous cyanide, carefully raise the temperature to 160°C, react for 6h to the end of the reaction, filter off the solid, and get 2,3-dichlorobenzoyl cyanide solution;
[0043] (2) Condensate preparation: Add 400g of aminoguanidine carbonate and 2L of methanol into the reactor, stir and add 300g of concentrated sulfuric acid dropwise, the entrainer is evaporated after the addition is completed, and the water is taken out, suction filtered, and the solid enters the reaction bottle , stirred at 80°C and dried under reduced pressure. Add the 2,3-dichlorobenzoyl cyanide solution obtained in step (1), raise the temperature to 80°C and react for 3...
Embodiment 3
[0047] A kind of improved lamotrigine synthetic technique, concrete implementation steps are as follows:
[0048] (1) Synthesis of 2,3-dichlorobenzoyl cyanide: Add 1kg of 2,3-dichlorobenzoic acid and 0.5kg of thionyl chloride to the reactor, react for 7h to the end, and distill the oxychloride under reduced pressure sulfone, then add 0.5kg cuprous cyanide, carefully raise the temperature to 160°C, react for 6h to the end of the reaction, filter out the solid, and get 2,3-dichlorobenzoyl cyanide solution;
[0049] (2) Condensate preparation: Add 0.8kg aminoguanidine carbonate and 3.5L toluene into the reactor, stir and add 0.6kg concentrated sulfuric acid dropwise, after the dropwise addition is completed, the entrainer is evaporated, and water is taken out, suction filtered, solid into the reaction flask, stirred at 80°C and vacuum-dried. Add the 2,3-dichlorobenzoyl cyanide solution obtained in step (1), raise the temperature to 80°C and react for 4 hours to the end of the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com