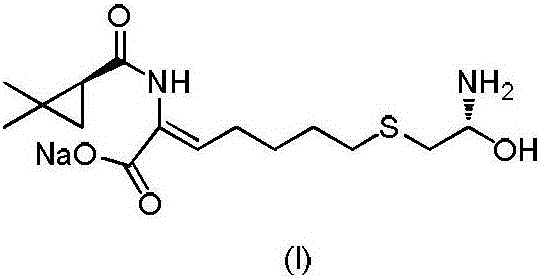
Preparation method of 7-chlorin-(1-oxo ethyl) ethyl oenanthate
A technology of oxoethyl and ethyl heptanoate, which is applied to the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc., which can solve the problem of increased energy consumption due to reaction time, unsuitability for industrial production, and insufficient environmental friendliness and other problems, to achieve the effect of shortening the response time, shortening the response time, improving convenience and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
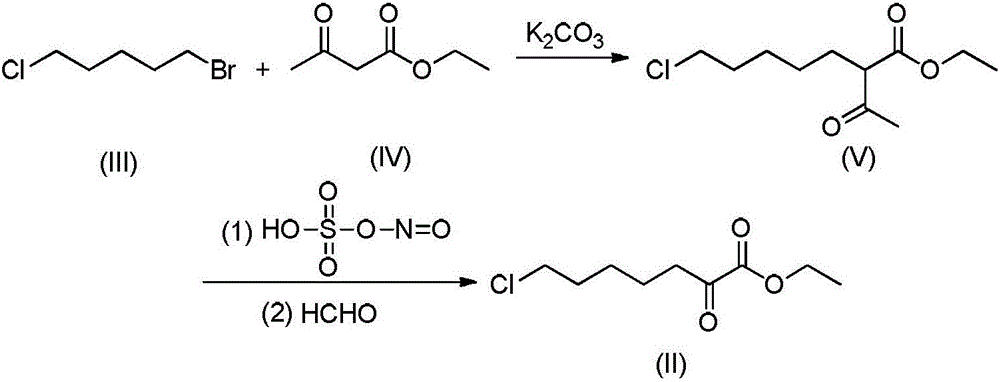
[0039] The invention provides a kind of preparation method of 7-chloro-(1-oxoethyl) ethyl heptanoate, comprising the steps:
[0040] Using 1-bromo-5-chloropentane of structural formula (III) and ethyl acetoacetate of structural formula (IV) as starting materials, in the presence of a polar solvent, an acid-binding agent and a phase-transfer catalyst, heating at normal pressure to the The polar solvent was boiled and reacted for 6 to 8 hours; the insolubles were removed by filtration, and the filtrate was concentrated under reduced pressure to obtain 7-chloro-(1-oxoethyl) ethyl heptanoate (crude product) of structural formula (V). The polar solvent is recovered and applied mechanically in the next batch of reactions.
[0041] The molar ratio of 1-bromo-5-chloropentane to ethyl acetoacetate is 0.8-1.0:1.0-1.3, preferably 0.9-0.95:1.0-1.05.
[0042]The phase transfer catalyst is selected from tetrabutylammonium bromide, tetrabutylammonium chloride, benzyltrimethylammonium chlori...
Embodiment 1
[0051] Example 1 Preparation of ethyl 7-chloro-(1-oxoethyl)heptanoate
[0052] At room temperature, 16.7g (0.09mol) of 1-bromo-5-chloropentane, 13.0g (0.10mol) of ethyl acetoacetate and 6.4g (0.025mol) of polyethylene glycol-400 (PEG-400) were dissolved in 100ml of ethyl acetate was stirred evenly, and 20.8g (0.15mol) of anhydrous potassium carbonate was added to the reaction solution. After the addition, the temperature was raised to 75-80°C, and the reaction was stirred while maintaining the temperature. The reaction progress was monitored by HPLC, and the reaction was complete in about 6.5 hours. After the reaction is complete, stop heating, cool the reaction mixture to room temperature, filter to remove solid insolubles, wash the solid residue with a small amount of ethyl acetate, collect the filtrate, and concentrate under reduced pressure to recover ethyl acetate (the next batch of reactions can be applied mechanically) to obtain light yellow The oil is the target pro...
Embodiment 2
[0053] Example 2 Preparation of ethyl 7-chloro-(1-oxoethyl)heptanoate
[0054] At room temperature, 20.2g (0.11mol) of 1-bromo-5-chloropentane, 15.6g (0.12mol) of ethyl acetoacetate and 14.4g (0.036mol) of polyethylene glycol-400 (PEG-400) were dissolved in 110ml of 2-methyltetrahydrofuran was stirred evenly, and 22.0g (0.22mol) of potassium bicarbonate was added to the reaction solution. After the addition was completed, the temperature was raised to 80-85° C., and the reaction was stirred while maintaining the temperature. The reaction progress was monitored by HPLC, and the reaction was complete in about 6 hours. After the reaction is complete, stop heating, cool the reaction mixture to room temperature, filter to remove solid insolubles, wash the solid residue with a small amount of 2-methyltetrahydrofuran, collect the filtrate, concentrate under reduced pressure to recover 2-methyltetrahydrofuran (the next batch of reactions can be applied mechanically) , to obtain a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com