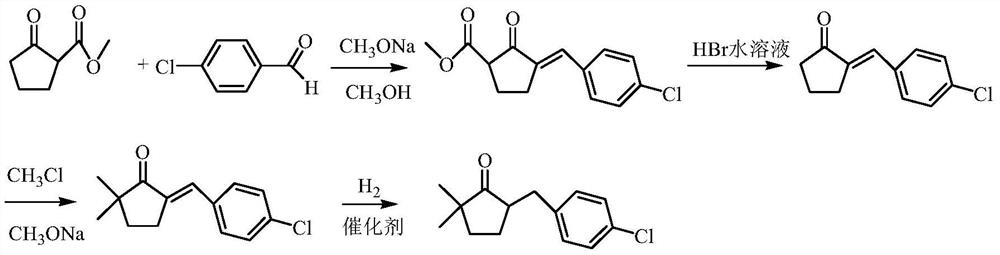
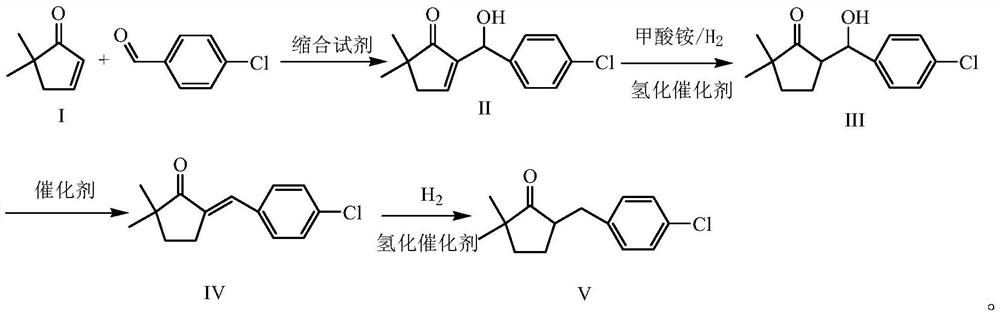
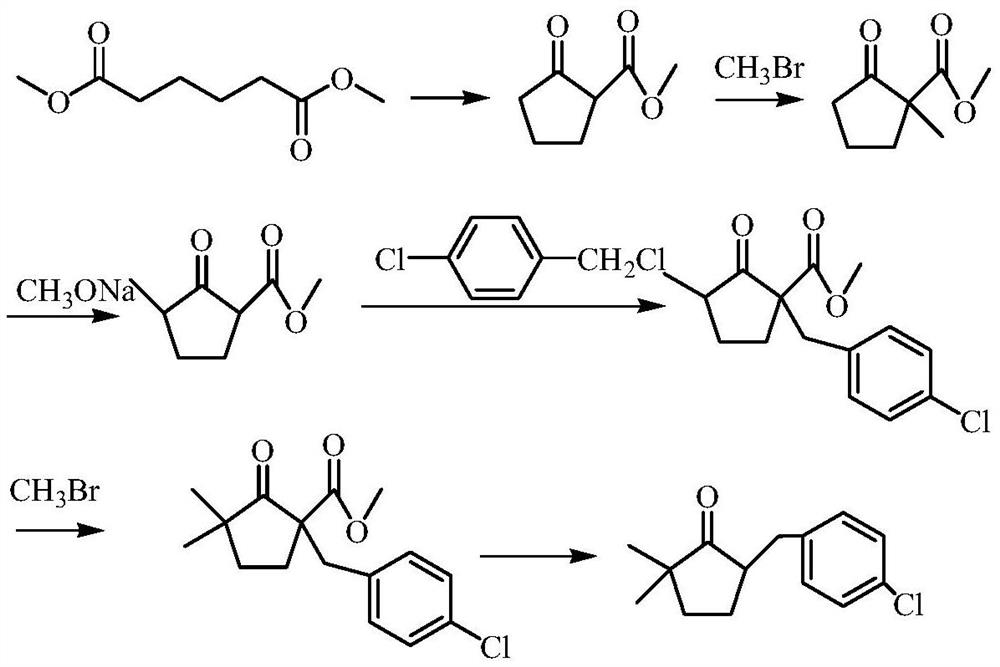
A kind of preparation method of 2,2-dimethyl-5-(4-chlorobenzyl) cyclopentanone
A dimethyl and chlorobenzyl technology, applied in the field of preparation of pesticide intermediates, can solve the problems of difficult operation, easy environmental pollution and high toxicity in post-processing, and achieves simple post-processing, ease of reaction, easy control, and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 A kind of preparation method of 2,2-dimethyl-5-(4-chlorobenzyl) cyclopentanone, concrete implementation is as follows:
[0033] 1) preparation of formula II compound
[0034] Add p-chlorobenzaldehyde (70.3g, 500mmol, 99.0%) and formula I compound 2,2-dimethyl-4-cyclopenten-1-one (60.2g, 537mmol) in the four-necked flask of 1L, then Add 500mL of methanol, stir, add DBU (40mL, 268mmol), react at room temperature for 3 hours, TLC shows that the raw material is basically converted completely, add 5% hydrochloric acid to acidify to a pH value of 3, concentrate, cool and crystallize, filter with suction, and use 50mL of the obtained solid to After rinsing with cold methanol and drying, the compound of Formula II (93.5 g, 374 mmol) was obtained as a light yellow solid with a purity of 96% and a yield of 88.1% (calculated as p-chlorobenzaldehyde).
[0035] 2) preparation of formula III compound
[0036] Add formula II compound (31.5g, 126mmol) in 1L four-necked f...
Embodiment 2
[0041]Embodiment 2 A kind of preparation method of 2,2-dimethyl-5-(4-chlorobenzyl) cyclopentanone, concrete implementation is as follows:
[0042] 1) preparation of formula II compound
[0043] Add p-chlorobenzaldehyde (70.3g, 500mmol, 99.0%) and formula I compound 2,2-dimethyl-4-cyclopenten-1-one (61.6g, 550mmol) in the four-necked flask of 1L, then Add 500mL of methanol, stir, add DBU (25mL, 167mmol), react at room temperature for 4 hours, TLC shows that the raw material is basically converted completely, add 5% hydrochloric acid to acidify to pH value 3, concentrate, cool and crystallize, suction filter, the obtained solid is washed with 50mL After rinsing with cold methanol and drying, the compound of Formula II (92.5 g, 370 mmol) was obtained as a pale yellow solid with a purity of 97% and a yield of 87.2% (calculated as p-chlorobenzaldehyde).
[0044] 2) preparation of formula III compound
[0045] Add formula II compound (31.5g, 126mmol) in 1L four-necked flask, then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com