Catalyst containing palladium compound, preparation method and application thereof
A technology of palladium complexes and catalysts is applied in the synthesis method and application research field of high-efficiency palladium catalysts, which can solve the problems of in-depth exploration of catalytic activity, catalytic performance, and detection, and achieve good catalytic activity and high yield. , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Catalyst PdI 2 (PPh 3 ) 2 The synthesis steps are as follows:
[0036]
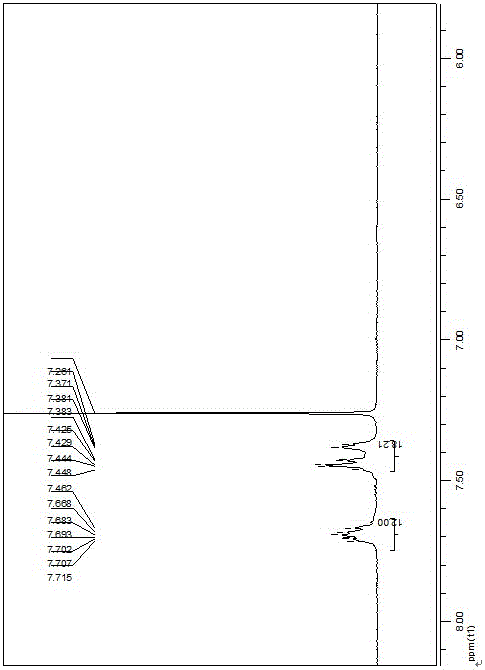
[0037] Under ice-bath conditions, iodomethane (712 mg, 5 mmol) was slowly dripped into a round-bottomed flask filled with dichloropyridine (568 mg, 5 mmol), and stirred while adding, after the addition was completed, return to room temperature and continue After stirring at room temperature for 3 h, a yellow precipitate precipitated out. After filtration, the precipitate was recrystallized with a methanol-ether mixed solvent to obtain a light yellow solid. Then take a dry 50 mL Schlenk bottle, add the product from the previous step and tetrakis(triphenylphosphine) palladium (2.08 g, 1.8 mmol), and after the mixture is evacuated several times with nitrogen, anhydrous toluene (20 mL) is added to the reaction flask , and then heated to 100 °C, the solution gradually turned orange-red during the reaction, and with the prolongation of the reaction time, a red solid appeared on the bottle wall. Af...
Embodiment 2
[0040] Catalyst PdBr 2 (PPh 3 ) 2 The synthesis steps are as follows:
[0041]
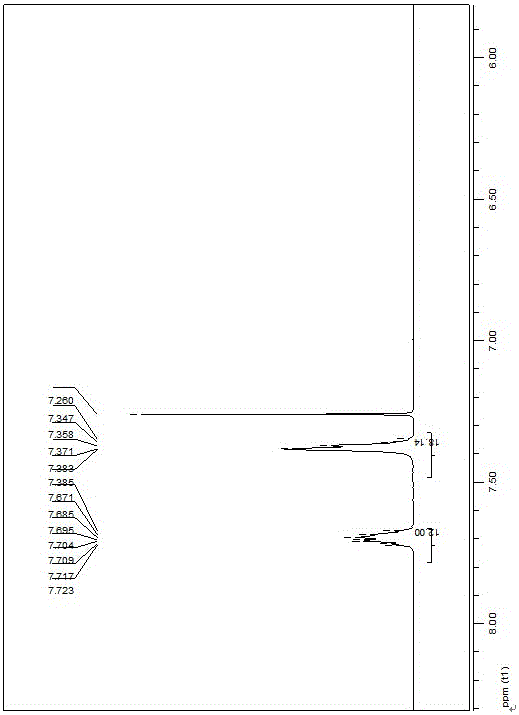
[0042] Add o-bromopyridine (530 mg, 3.4 mmol) and iodoacetic acid (432 mg, 2.3 mmol) into a dry 50 mL round bottom flask, mix and heat to 80 °C, stir for 4 h, return to room temperature, and add a large amount of diethyl ether , a yellow viscous substance precipitated out after stirring, and was allowed to stand overnight, and recrystallized from methanol to obtain a pale yellow solid. Then take a dry 50 mL Schlenk bottle, add the product from the previous step and tetrakis(triphenylphosphine) palladium (1.51 g, 1.3 mmol), vacuumize nitrogen, add glacial acetic acid (2 mL), and then heat to 100 ° C for 3 h Afterwards, the solution becomes orange-red, and it is purified through a silica gel column, and the orange-yellow ribbon is concentrated to obtain an orange-yellow solid that is the catalyst PdBr 2 (PPh 3 ) 2 , and further recrystallized from dichloromethane / petroleum ether to obtain P...
Embodiment 3
[0045] Catalyst PdI 2 (PPh 3 ) 2 The condition screening method and the results of the catalytic activity test applied in the Heck coupling reaction of p-bromonitrobenzene and ethyl acrylate are as follows:
[0046] Vacuum the reaction flask with nitrogen, and add PdI in sequence 2 (PPh 3 ) 2 (0.26mg, 0.3x10 -3 mmol), p-bromonitrobenzene (606mg, 3 mmol), ethyl acrylate (360mg, 3.6 mmol), base (455mg, 4.5 mmol), solvent (1mL), and stirred at constant temperature 110 ℃ for 12 h. After the reaction, cool to room temperature, wash with water, extract three times with dichloromethane, combine the organic layers, dry and concentrate, and purify through the column (petroleum ether:dichloromethane=1:1), collect the product band, concentrate and weigh, and calculate the yield .
[0047] The reaction results are shown in Table 1 below:
[0048] Table 1 Catalyst PdI 2 (PPh 3 ) 2 Condition screening results of the catalytic activity test applied to the Heck coupling reaction o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com