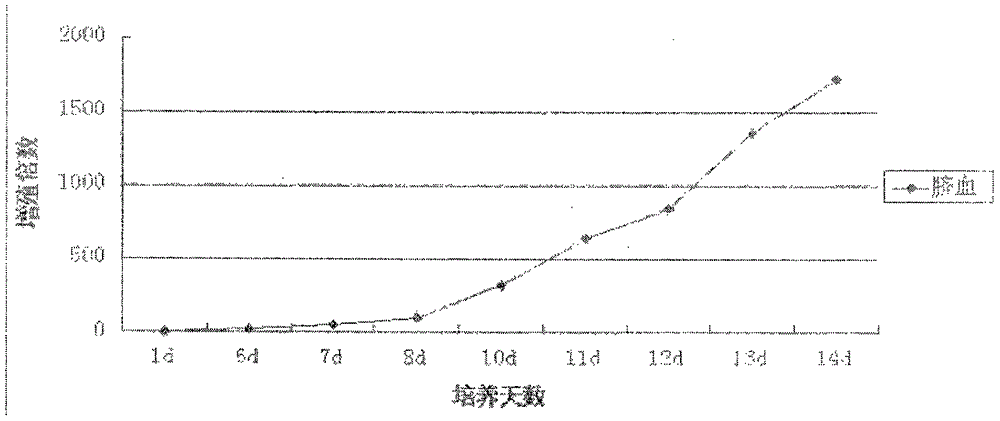
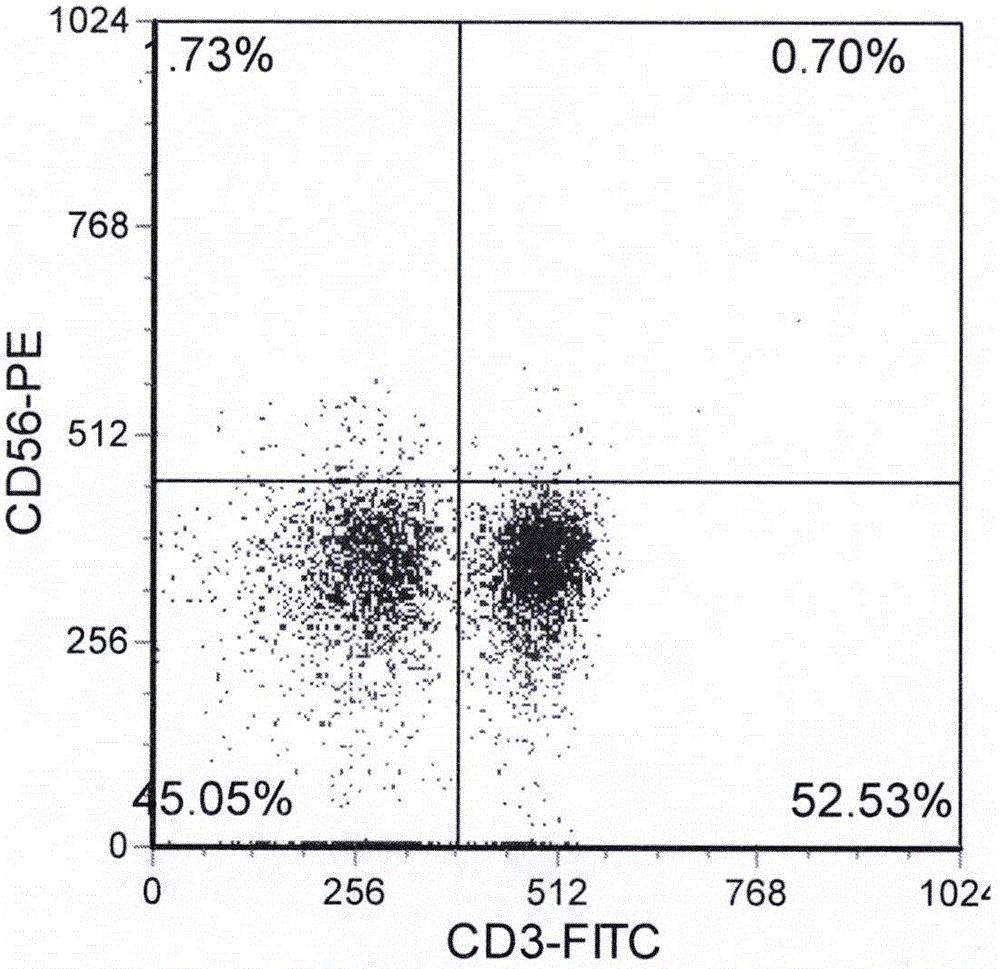
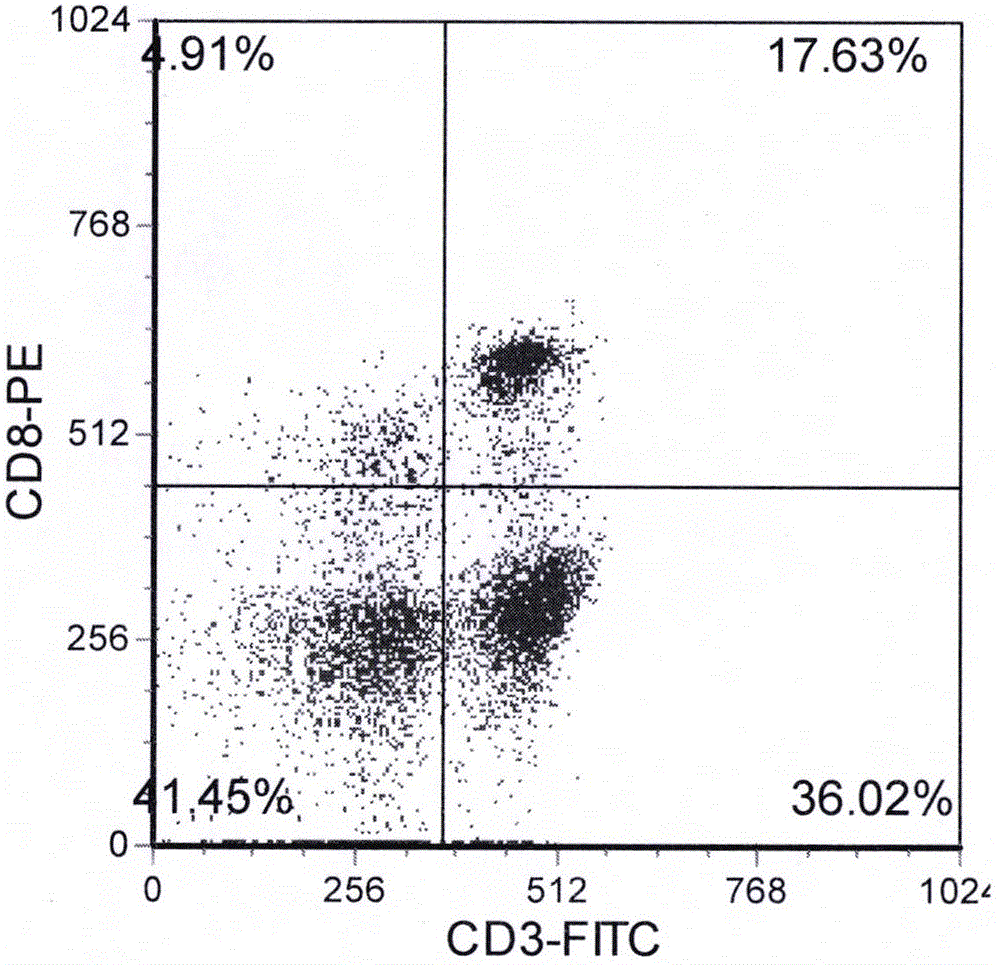
Method for culturing cord blood lymphocyte DC-CIK
A technology of DC-CIK and lymphocytes, which is applied in the direction of cell culture active agents, blood/immune system cells, tissue culture, etc., can solve the problem of CIK cell proliferation multiples and effective cell content, increased expression, and failure to take into account The effect of T regulatory cell content on tumor immune escape and other issues can be achieved to improve the activation efficiency and expansion efficiency, increase the expansion level, and increase the effect of killing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0055] The reagents used in this embodiment are as follows:
[0056] Umbilical cord blood was purchased from Shandong Qilu Stem Cell Engineering Co., Ltd.
[0057] Ficoll, purchased from GE company;
[0058] IL-2 was purchased from Beijing Tongli Haiyuan Biotechnology Co., Ltd.;
[0059] CD3mAb was purchased from Beijing Tongli Haiyuan Biotechnology Co., Ltd.;
[0060] GM-CSF was purchased from Beijing Tongli Haiyuan Biotechnology Co., Ltd.;
[0061] IL-4 was purchased from Beijing Tongli Haiyuan Biotechnology Co., Ltd.;
[0062] rhTNF-a was purchased from Beijing Tongli Haiyuan Biotechnology Co., Ltd.;
[0063] IFN-γ, purchased from Beijing Tongli Haiyuan Biotechnology Co., Ltd.;
[0064] CTLA-4, purchased from Beijing Tongli Haiyuan Biotechnology Co., Ltd.;
[0065] PD-1, purchased from Beijing Tongli Haiyuan Biotechnology Co., Ltd.;
[0066] GT-T551H3 medium was purchased from TAKARA Company.
[0067] The method used in this embodiment for amplifying DC-CIK:
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com