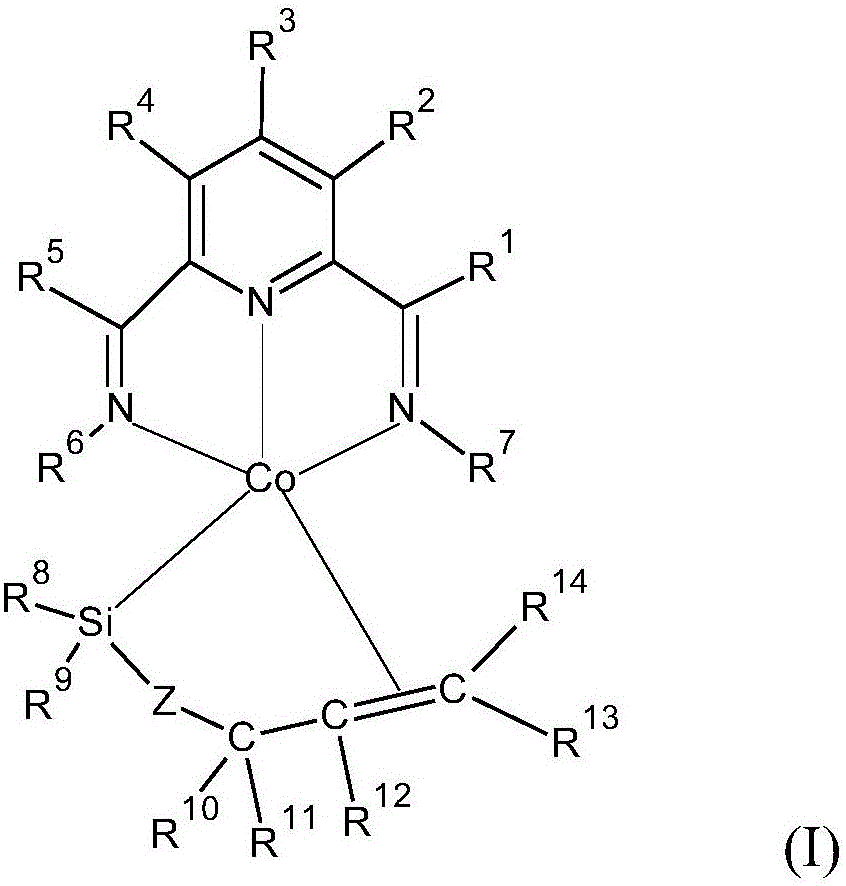
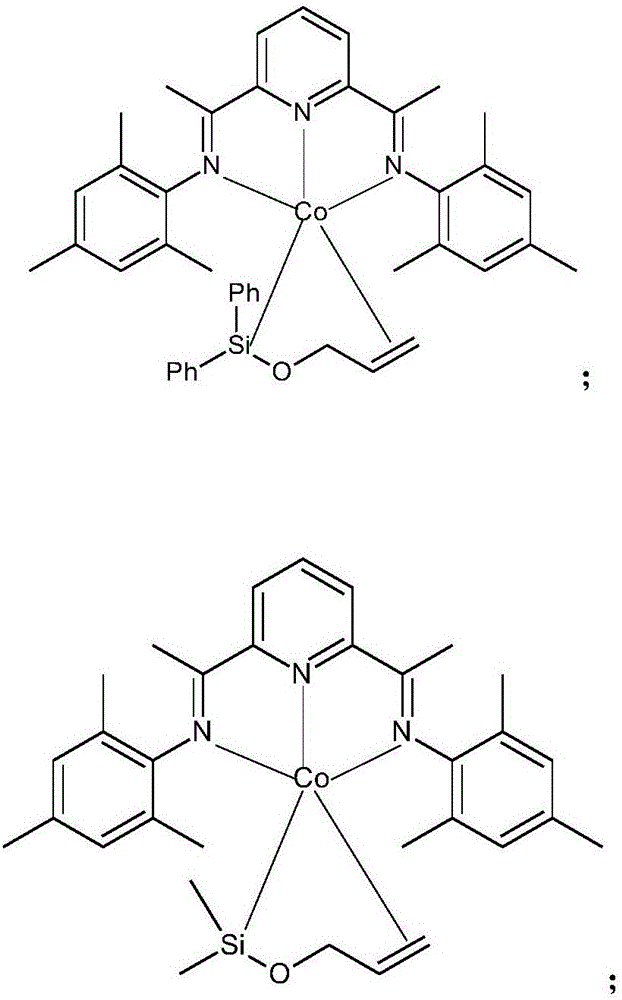
Cobalt catalysts and their use for hydrosilylation and dehydrogenative silylation
An alkyl, unsaturated technology, used in catalytic reactions, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problems of reduced catalytic performance, aggregation or agglomeration, low activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] Embodiment 1: the synthesis of allyloxydiphenylsilane (alloxydiphenylsilane)
[0141]
[0142] The synthesis of allyloxydiphenylsilane follows a modified literature procedure described by Bergens, S.H.; Noheda, P.; Whelan, J.; Bosnich, B.J.Am.Chem.Soc. Implemented in the air. Allyl alcohol (2.9 g, 50 mmol) and triethylamine (5.1 g, 50 mmol) were dissolved in Et in an ice bath 2 The solution in O (250 mL) was cooled. The solution was treated dropwise with diphenylsilane chloride (11 g, 50 mmol) with rapid stirring. A large fluffy white precipitate was immediately observed (Et 3 NHCl). The mixture was warmed to room temperature and stirred for 1 h. The resulting solution was filtered through Celite and washed with Et 2 O washing. The filtrate was concentrated and resulted in a colorless oil which upon distillation (84-85°C, 65mmTorr) gave the desired product in 80% yield as a colorless oil. 1 H NMR (400MHz, benzene-d 6 )δ7.74–7.62(m,4H),7.27–7.06(m,6H),5.81(...
Embodiment 2
[0143] Embodiment 2: the synthesis of allyloxydimethylsilane (alloxydimethylsilane)
[0144]
[0145] Allyloxydimethylsilane was prepared in a similar manner to allyloxydiphenylsilane. Allyl alcohol (2.9 g, 50 mmol) and triethylamine (5.1 g, 50 mmol) were dissolved in Et in an ice bath 2 The solution in O (250 mL) was cooled. With rapid stirring, dimethylsilyl chloride (4.7 g, 50 mmol) was added dropwise to the solution. A large fluffy white precipitate was immediately observed (Et 3 NHCl). The mixture was warmed to room temperature and stirred for 1 h. The resulting solution was filtered through celite and washed with Et 2 O washing. The filtrate was concentrated and produced a colorless oil, which was then fractionally distilled (79-83 °C) to provide the product as a colorless oil in 50% yield. 1 H NMR (400MHz, chloroform-d) δ5.93 (ddt, J = 17.2, 10.1, 5.0Hz, 1H), 5.26 (dq, J = 17.2, 1.8Hz, 1H), 5.12 (dq, J = 10.4, 1.6 Hz, 1H), 4.68–4.55 (m, 1H), 4.18 (dt, J=5.0...
Embodiment 3
[0146] Embodiment 3: ( Mes PDI)Co(Ph 2 SiOC 3 H 5 )Synthesis
[0147]
[0148] In the glove box, place in 1mL Et 2 O in ( Mes PDI) a purple solution of CoMe (47mg, 0.1mmol) and Et of allyloxydiphenylsilane (24mg, 0.1mmol) 2 The O (1 mL) solution was mixed and stirred for 0.5 h. A maroon solution was observed. The solution was filtered through celite and concentrated under vacuum. The resulting solution was layered with pentane and stored at -35°C for 2 days and maroon crystals resulted. 1 H NMR (400MHz, benzene-d 6 )δ7.77(d,J=7.6Hz,1H),7.70(d,J=7.6,1H),7.31(t,J=7.6Hz,1H),7.10–6.94(m,8H),6.88–6.82 (m,2H),6.73–6.64(m,2H),6.62(s,1H),6.57(s,1H),5.24(dd,J=9.9,5.4Hz,1H),4.78(br,1H), 3.49(t, J=10.4Hz, 1H), 3.16(d, J=8.7Hz, 1H), 2.72(d, J=12.5Hz, 1H), 2.05(s, 3H), 2.01(s, 3H), 1.87(s,3H),1.76(s,3H),1.65(s,3H),1.44(s,3H),1.42(s,3H)1.02(s,3H). 13 C NMR (126MHz, benzene-d 6 )δ154.33,150.22,149.42,148.74,147.28,146.88,144.56,140.38,137.90,134.80,134.69,13...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com