Controlled-release preparation containing metformin hydrochloride and glimepiride and preparation method thereof
A technology of metformin hydrochloride and controlled-release tablets, which is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, and pharmaceutical formulations, which can solve the problem of uncontrolled drug release, loss of anti-environmental interference, and inconsistent release degrees. Consistency and other problems, to achieve the effect of good release regulation and improved release uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
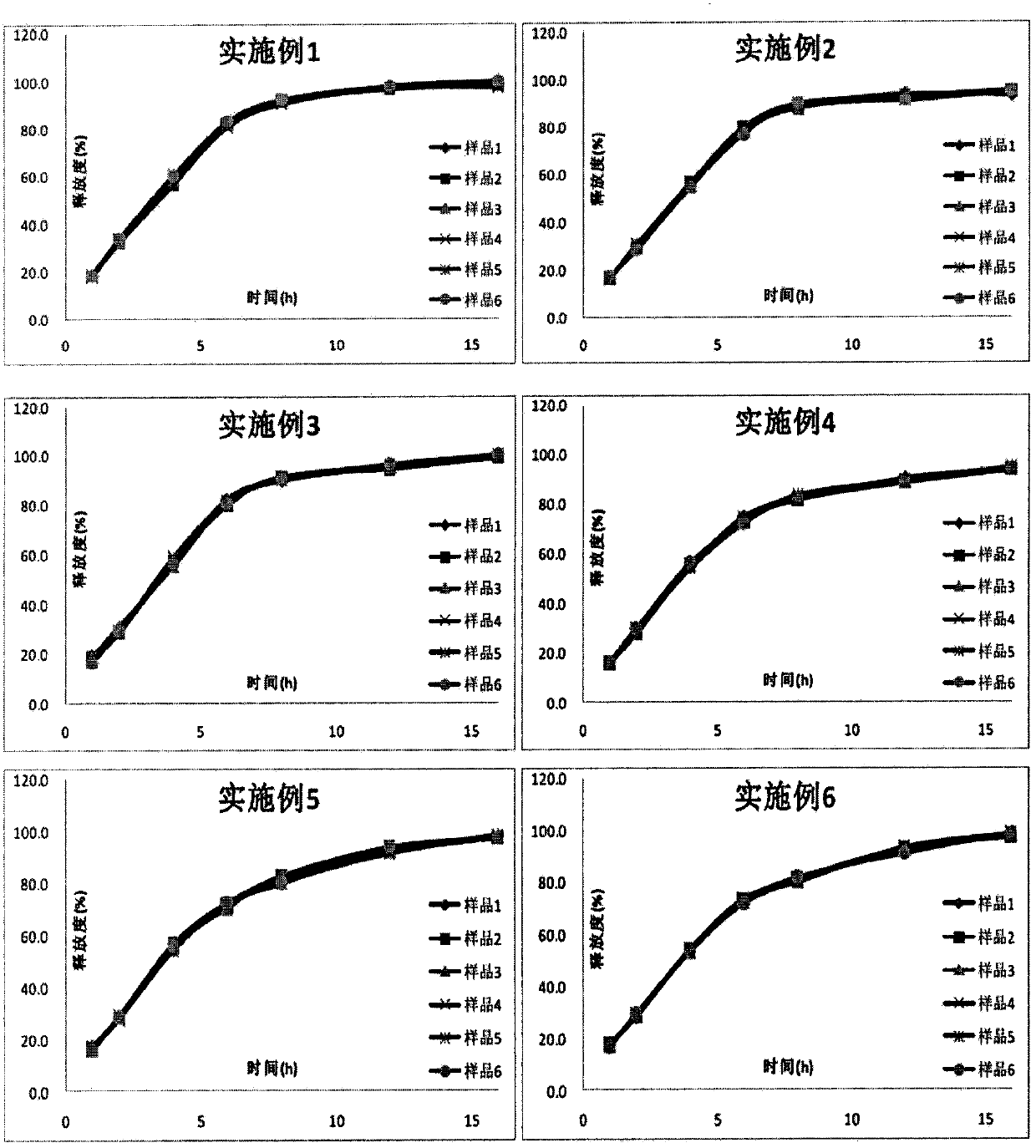
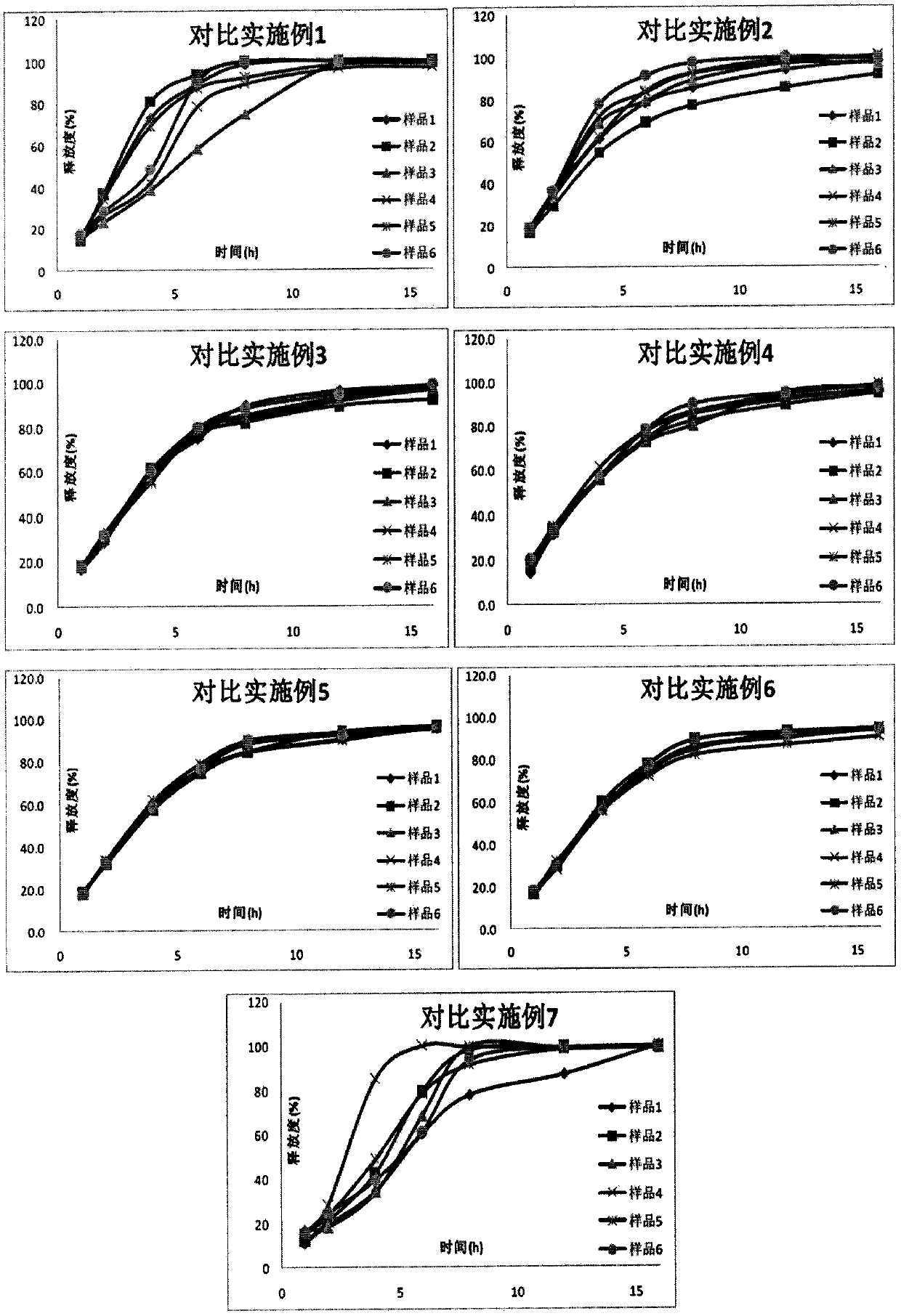
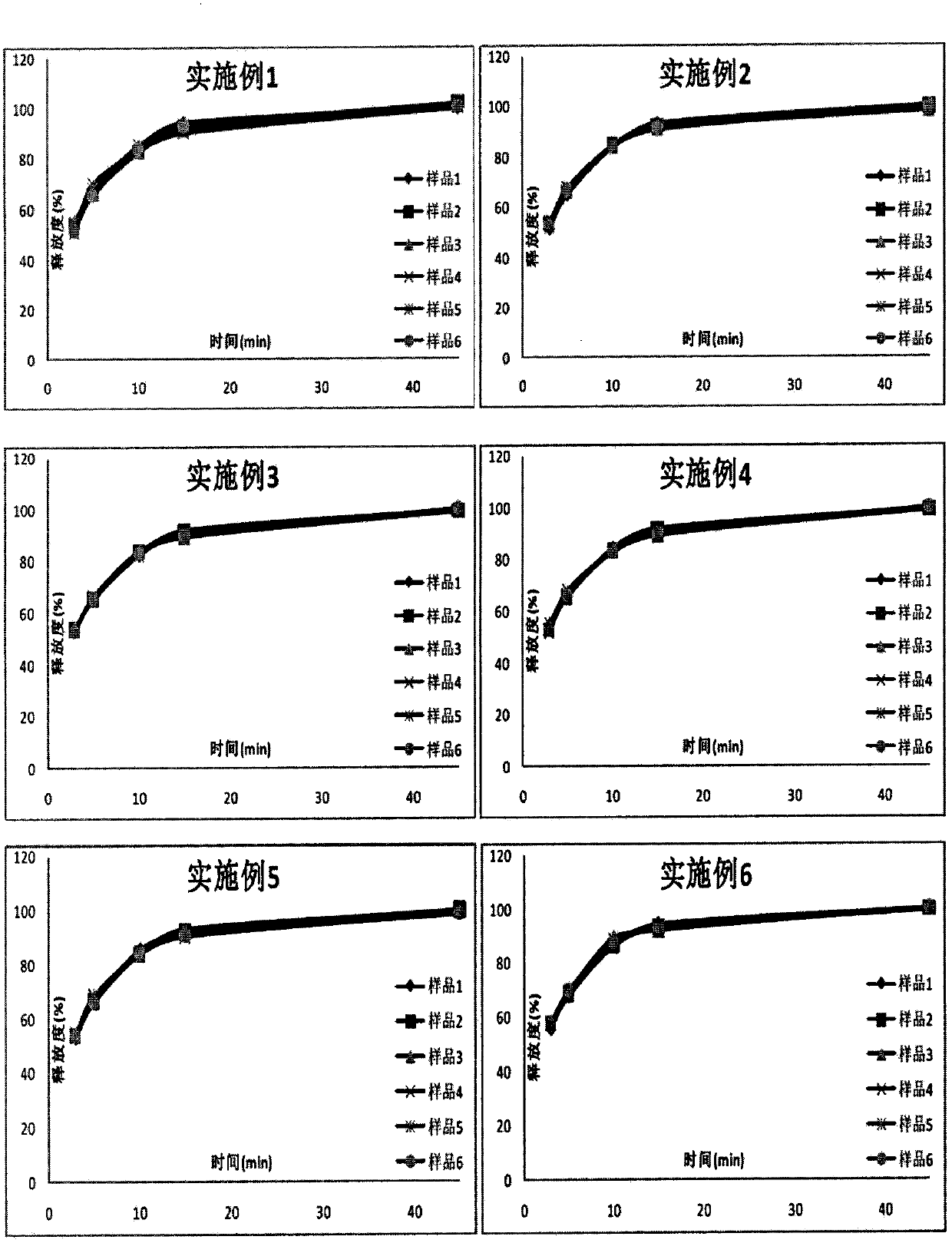
Examples
Embodiment 1
[0072] Tablet core composition (mg / P)
[0073]
[0074]
[0075] Composition of controlled release coating layer (mg / P)
[0076] Cellulose acetate 32
[0077] Polyethylene glycol 1500 8
[0078] Acetone 800
[0079] Composition of drug-loaded coating layer (mg / P)
[0080]
[0081] Composition of film coating layer (mg / P)
[0082] Coating material 10
[0083] 50% alcohol 100
[0084] Granulation: powder metformin hydrochloride through a 100-mesh sieve, mix evenly with other excipients except magnesium stearate, use 95% ethanol to make soft material, 30-mesh granulation, dry below 50°C, control moisture below 2.0%, Sieve through a 20-mesh sieve, add lubricant magnesium stearate and mix well;
[0085] Tablet compression: use a shallow concave die to compress the tablets, set the rotary tablet press to compress the tablets, and control the hardness to 10-20kg / m2.
[0086] Semi-permeable membrane: Take a certain amount of acetone, add cellulose acetate and PEG3350 ...
Embodiment 2
[0091] Tablet core composition (mg / P)
[0092]
[0093] Composition of drug-loaded coating layer (mg / P)
[0094]
[0095] 1, semi-permeable membrane layer and film coating composition are the same as embodiment 1;
[0096] 2. The production process is the same as in Example 1.
Embodiment 3
[0098] Tablet core composition (mg / P)
[0099]
[0100] Composition of drug-loaded coating layer (mg / P)
[0101]
[0102] 1, semi-permeable membrane layer and film coating composition are the same as embodiment 1;
[0103] 2. The production process is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com