Method for establishing pseudomonas aeruginosa infectious pneumonia animal model
A Pseudomonas aeruginosa, animal model technology, applied in pharmaceutical formulations, compound screening/testing, medical raw materials derived from bacteria, etc., can solve the problem that cannot represent the prevalence of PA strains, it is difficult to carry out experiments in large quantities, and the death of experimental animals and other problems to achieve the effect of avoiding the death of experimental animals, being representative, and easy to infect conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: preparation of Pseudomonas aeruginosa bacterium liquid
[0039] Take the frozen Pseudomonas aeruginosa clinical strain XN-1, revive it with LB nutrient agar plate, and culture it aerobically at 37°C overnight. Pick a single colony and inoculate 20mL of LB liquid medium, and incubate with aerobic shaking at 180rpm for 15 hours at 37°C, then inoculate 0.2mL into 20mL of LB liquid medium, and culture with aerobic shaking at 230rpm for 6h at 37°C. The cells were collected by centrifugation at 6000g, washed twice with normal saline, and then resuspended with normal saline. The OD of the bacterial solution was measured by a spectrophotometer 600 Value, and adjust the OD value to 1.0 with normal saline, through serial dilution, and count on LB plates to obtain Pseudomonas aeruginosa at OD=1.0 when it is 6.0×10 9 CFU / ml.
Embodiment 2
[0040] Example 2: Establishment of mouse anesthesia and nasal drop scheme
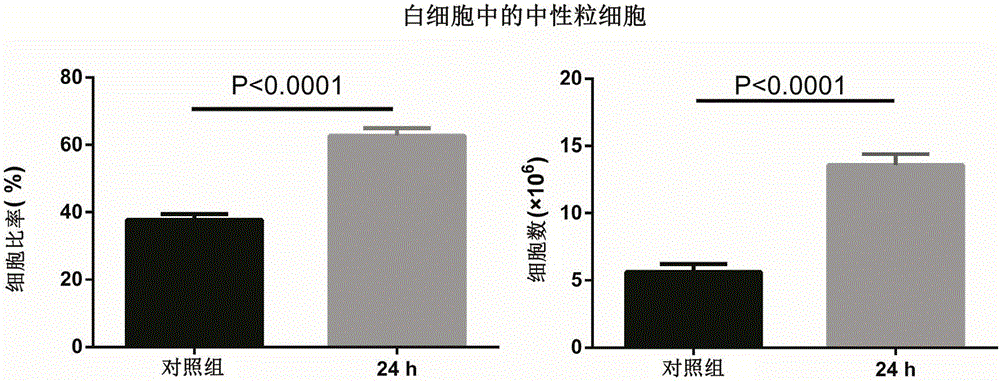
[0041]In the infection experiment of the Pseudomonas aeruginosa pneumonia model, in order to ensure the smooth progress of the nasal drip process, the mice were first anesthetized with isoflurane. Anesthesia method: Inhalation induction anesthesia with 5% isoflurane using oxygen as the transport carrier, and maintain anesthesia with 3% concentration after the mice enter the surgical deep anesthesia period, this method can achieve better anesthesia effect. The control of the nasal drop dose is the guarantee of the stability of the model. In order to better control the nasal drop dose and prevent the bacterial liquid from entering the mouth and generating air bubbles during the nasal drop process, the following measures have been taken: (1) Since the nasopharynx has a physiological The more the head is tilted, the easier it is for nasal drops to enter the mouth. Therefore, during the nasal dripping proce...
Embodiment 3
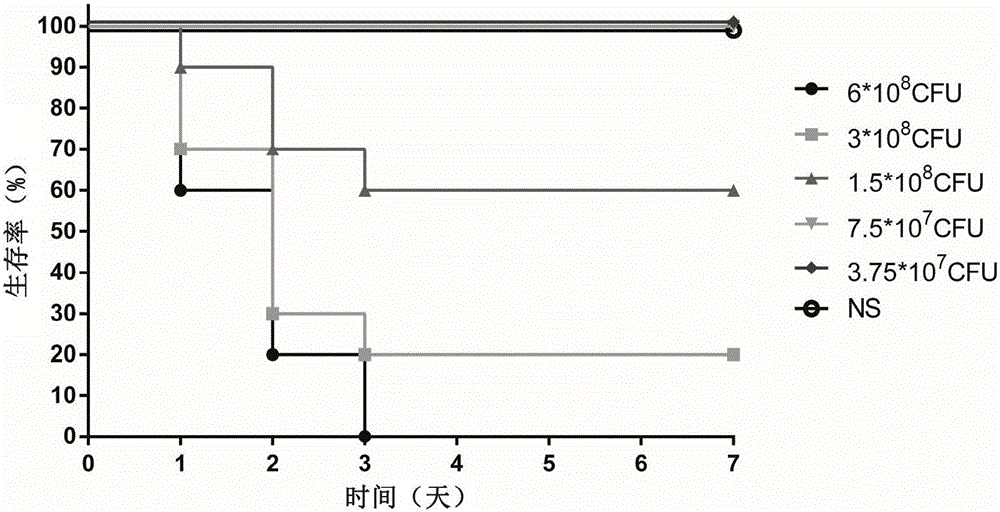
[0042] Example 3: Determination of Infection Dose
[0043] Physiological saline was used to adjust the PAXN-1 bacterial solution obtained in Example 1 to five different concentrations, and the experimental animal female BALB / c mice were randomly divided into 5 groups, and the mice were anesthetized with isoflurane and infected by nasal drops , the infection dose for each mouse was 20 μL, and the same dose of normal saline (NS) was used as a blank control. The grouping and infection status of the animals are shown in Table 1. After the infection, the death of the mice was observed every other day, and the observation period was 7 days. After the observation period, the remaining animals were treated with CO 2 Euthanized by inhalation.
[0044] Table 1: Determination of Infection Dose in Pseudomonas Aeruginosa Pneumonia Model
[0045] Group Nasal drip Dose (CFU) quantity Concentration (CFU / ml) Dropping amount (μl) 1 PAXN-1 6.0×10 8 10 3.0×10 10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com