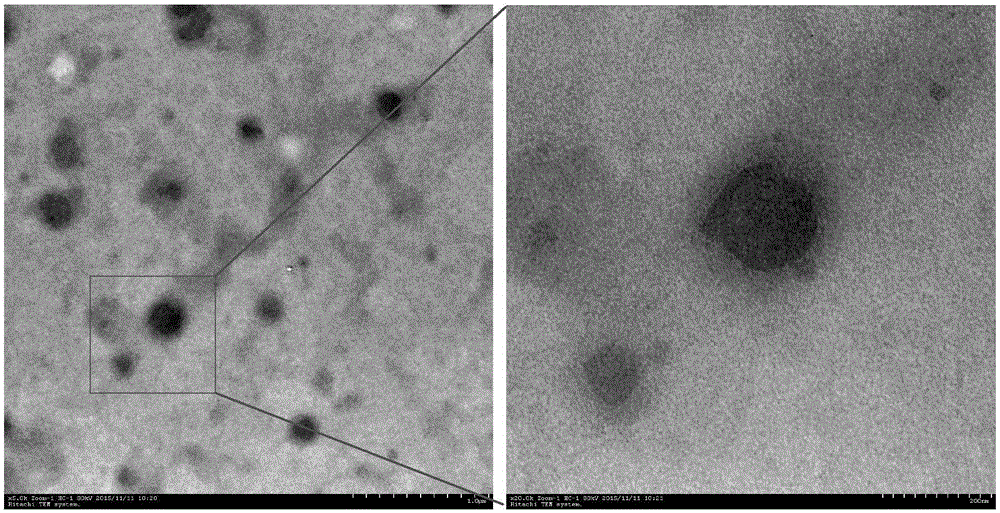
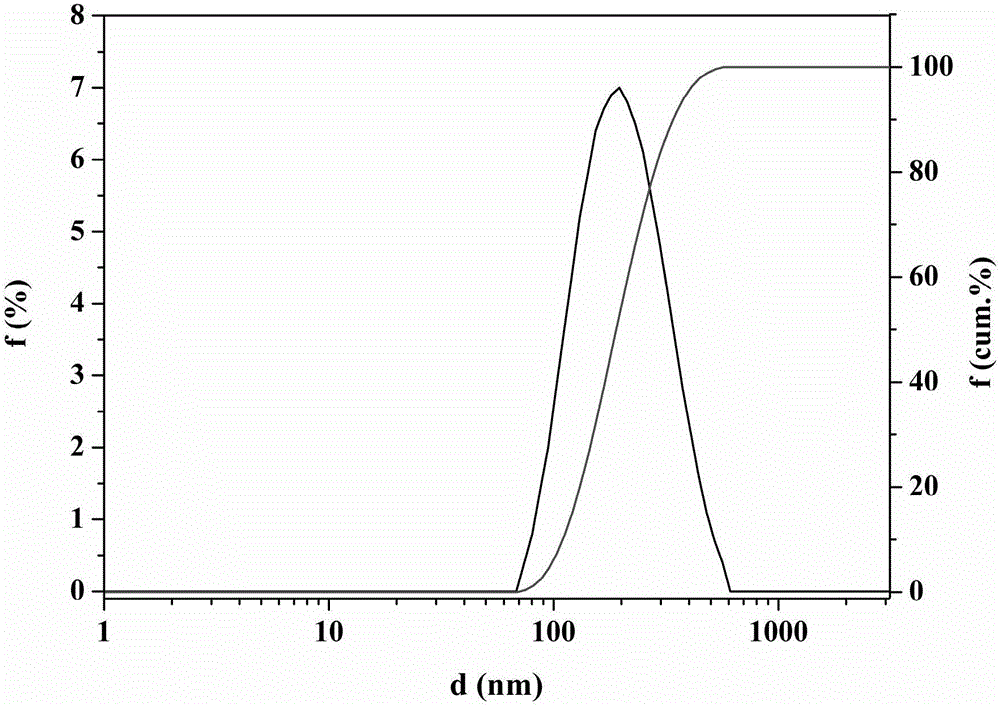
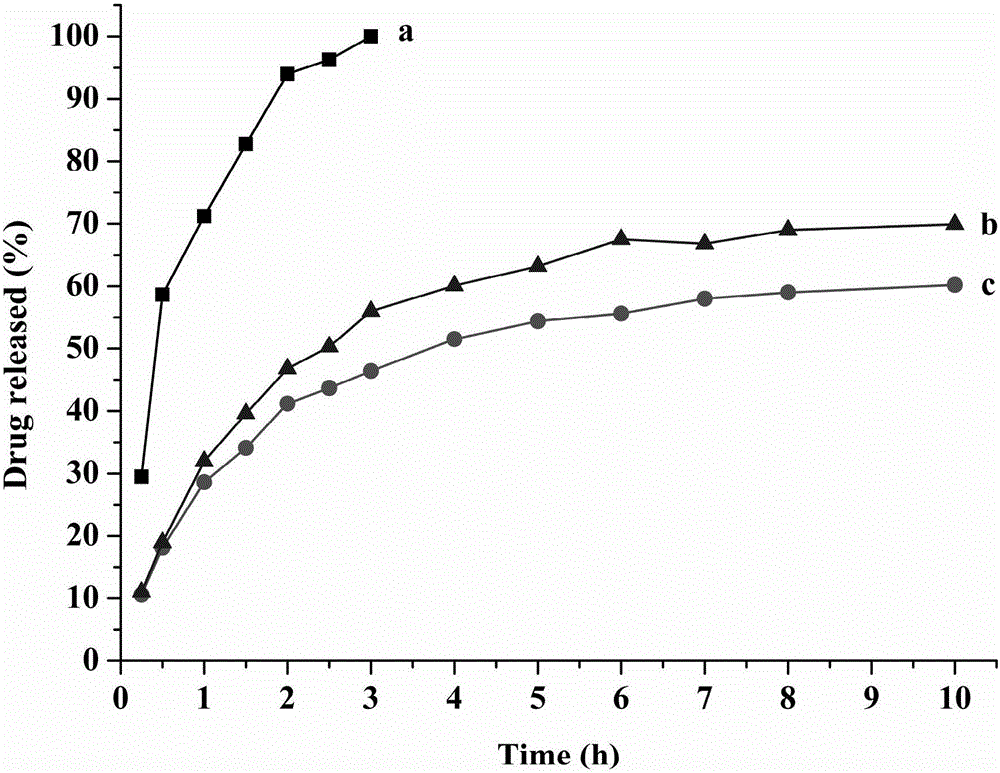
Montmorillonite inlaid liposome preparation and preparation method thereof
The technology of montmorillonite lipid and montmorillonite is applied in the field of liposome preparation inlaid with montmorillonite and its preparation, and can solve the problem of reducing the toxic and side effects of drug bioavailability, low drug bioavailability and poor corneal permeability and other problems to achieve the effect of improving bioavailability, improving bioavailability and side effects, and slowing down the release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] A kind of preparation method of mosaic montmorillonite liposome preparation, with drug-loaded montmorillonite, phospholipid, cholesterol, antioxidant, octadecylamine as oil phase, ammonium sulfate solution as water phase, prepare liposome, drug-loaded The montmorillonite is loaded into the liposome, and then the liposome is incubated with the drug to obtain the montmorillonite-embedded liposome preparation.
[0028] A preparation method of mosaic montmorillonite liposome preparation, comprising the following steps:
[0029] 1) Adsorbing drugs with montmorillonite to prepare drug-loaded montmorillonite;
[0030] 2) Dissolve drug-loaded montmorillonite, phospholipids, cholesterol, octadecylamine, and antioxidants in an organic solvent to form an organic phase; use ammonium sulfate solution as the water phase;
[0031] 3) Heat the organic phase and the water phase to 40-80°C respectively, slowly inject the organic phase into the water phase under stirring conditions, and ...
Embodiment 1
[0053] (1) Place the montmorillonite in 5v / v% sulfuric acid, acidify it in a water bath at 70°C for 0.5h, wash it with double distilled water until neutral, treat it with ultrasonic to reduce the particle size, and obtain the acidified montmorillonite after drying, namely Acid-activated modified montmorillonite;
[0054] Prepare an aqueous solution of betaxolol hydrochloride (BH) with a concentration of 3 g / L, add acidified montmorillonite whose weight is 1 / 3 of the weight of BH, adjust the pH to 4, absorb in a water bath at 50 ° C for 6 h, and centrifuge and dry to obtain the loaded Medicinal montmorillonite (montmorillonite-betaxolol hydrochloride complex);
[0055] (2) Weigh 5 mg of drug-loaded montmorillonite, 150 mg of lecithin, 25 mg of cholesterol, 10 mg of octadecylamine and 3 mg of vitamin E and place them in a stoppered test tube, add an appropriate amount of absolute ethanol (so that the concentration of drug-loaded montmorillonite is 1~3mg / mL), after the cholester...
Embodiment 2
[0064] (1) Put the montmorillonite in 5v / v% sulfuric acid, acidify it in a water bath at 70°C for 0.5h, wash it with double distilled water until neutral, reduce the particle size by ultrasonic, and obtain the acidified montmorillonite after drying;
[0065] Prepare an aqueous solution of betaxolol hydrochloride (BH) with a concentration of 3g / L, add acidified montmorillonite with a weight of BH1 / 3, adjust the pH to 4, absorb in a water bath at 50°C for 6h, and centrifuge and dry to obtain the drug-loaded montmorillonite Tricholithiasis (montmorillonite-betaxolol hydrochloride complex);
[0066] (2) Weigh 15 mg of drug-loaded montmorillonite, 150 mg of lecithin, 25 mg of cholesterol, 10 mg of octadecylamine, and 5 mg of BHT (2,6-di-tert-butyl-4-methylphenol) into a stoppered test tube, Add an appropriate amount of absolute ethanol (so that the concentration of the drug-loaded montmorillonite is 1-3 mg / mL), after the cholesterol and stearylamine are dissolved, disperse the mont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com