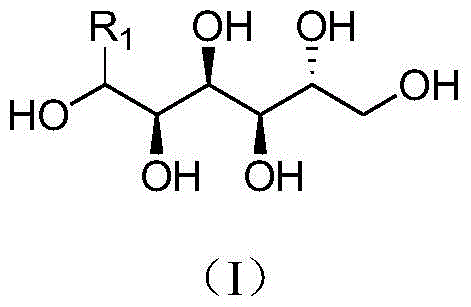
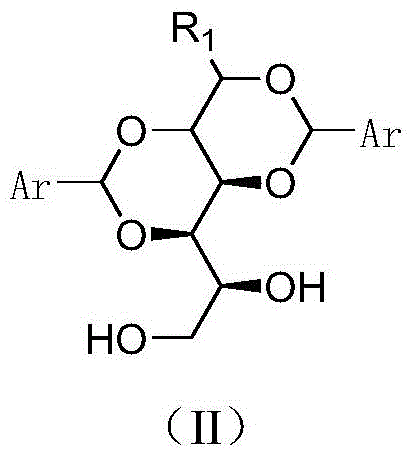
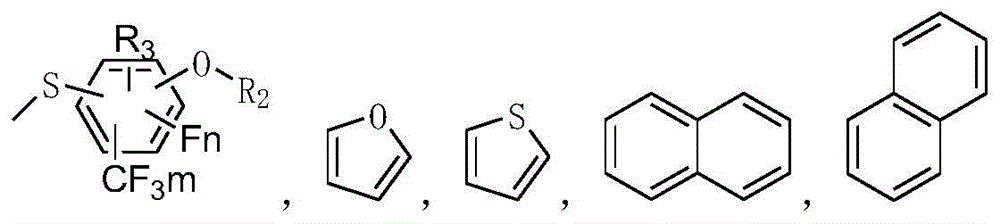
Sorbitol derivatives and preparing method and application of DBS polyolefin nucleating agent synthesized with sorbitol derivatives
A technology of sorbitol and derivatives, applied in the field of sorbitol derivatives and preparation thereof, can solve the problems of high odor, low transparency and low nucleation efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of 1-(2-butenyl)sorbitol:
[0057] D-glucose (5.12mmol) was dissolved in 50mL of water, 7.21mmol of indium powder or tin powder or gallium powder was added, 1-bromo-2-butene (3mL, 36.4mmol) was added dropwise, and after the reaction was stirred overnight, the aqueous phase After extracting both sides with ethyl acetate, extract with n-butanol four times, collect the n-butanol extract, and distill under reduced pressure to obtain 1-(2-butenyl)sorbitol with a yield of 70%.
[0058] 1 HNMR (CHCl 3 d 3)δppm: 5.70 (m, 1H, olefin H), 5.03 (m, 1H, olefin terminal H), 4.97 (m, 1H, olefin terminal H), 3.81 (m, 1H), 3.56 (m, 1H) , 3.38(m,1H), 3.37(m,3H), 3.29(m,1H), 1.96(m,2H), 1.48(m,2H).
Embodiment 2
[0060] Synthesis of 1-vinylsorbitol
[0061] D-glucose (10mmol) was dissolved in 100mL of anhydrous THF, and under the protection of nitrogen, the reaction solution was lowered to -78°C, and the ether solution of vinylmagnesium bromide (10mmol, 1M) was added dropwise to the reaction solution, and the temperature of the reaction solution was slowly raised After reaching room temperature and stirring for 2 hours, saturated ammonium chloride (100 mL) was added dropwise to quench the reaction, the aqueous phase was extracted twice with ethyl acetate, and extracted 4 times with n-butanol, and then distilled under reduced pressure to obtain 1-vinyl Sorbitol, yield 50%.
[0062] 1 HNMR (CHCl 3 d 3 )δppm: 5.89 (m, 1H, olefin H), 5.24 (m, 1H, olefin terminal H), 5.23 (m, 1H, olefin terminal H), 3.98 (m, 1H), 3.81 (m, 1H) ,3.56(m,1H),3.41(m,1H),3.38(m,1H),3.37(m,2H).
Embodiment 3
[0064] Synthesis of 1-butyl sorbitol:
[0065] 1-(2-butenyl)sorbitol (2.36g, 10mmol) was dissolved in 50mL of methanol, 0.236g of wet palladium carbon (10%w) was added to the reaction solution, and the reaction solution was replaced with hydrogen, reacted for 2 hours, and filtered Palladium carbon was removed, and methanol was distilled under reduced pressure to obtain 1-butyl sorbitol with a yield of 95%.
[0066] 1 HNMR (CHCl 3 d 3 )δppm: 3.81(m,1H), 3.56(m,1H), 3.38(m,1H), 3.37(m,3H), 3.29(m,1H), 1.44(m,2H), 1.33(m,2H ), 1.29(m,2H), 0.96(m,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com