Aconitine injection for intrathecal injection and preparation method thereof
A technology of intrathecal injection and aconitine, which is applied in the field of medicine, can solve the problems of difficulty in producing qualified products, excessive degradation of related substances, and low therapeutic index, and achieve rapid onset, small dose, and good analgesic effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
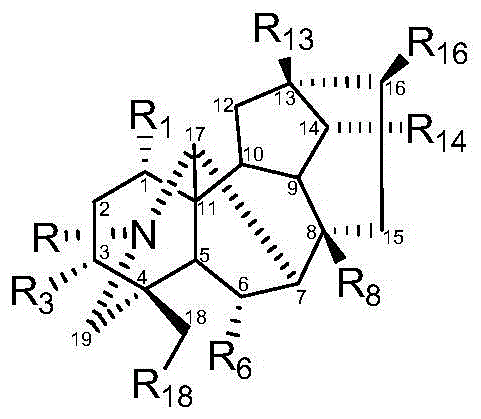
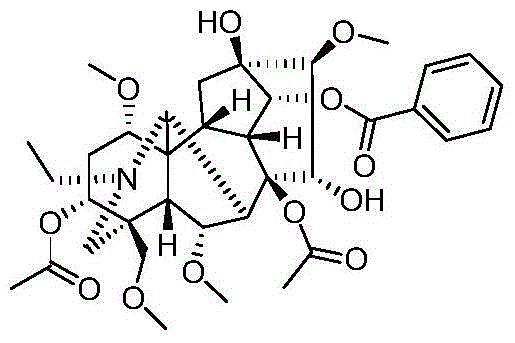
Image
Examples
Embodiment 1
[0050] Embodiment 1. Aconitin A injection prescription consists of (1000):
[0051] Aconitin 0.04g
[0052] Hydrobromic acid (47%) 0.011g
[0053] Water for injection 500ml
[0054] The preparation process of Aconitin A injection of the present invention is as follows: under 100-level laminar flow conditions, add 400ml of water for injection at 20-49°C in a beaker, carefully add 0.010g of hydrobromic acid (47%) and stir evenly under heat preservation , add 0.04g of aconitin, continue to stir until dissolved, and then add 0.001g of hydrobromic acid (47%) dissolved in 10ml of water for injection at 20 to 49°C under stirring to adjust the pH to 4.5 to 6.0, filter, and add 20 Water for injection at ~49°C to a total volume of 500ml, filtered through a 0.2μm membrane, and aseptically filled into 0.5ml / bottle, to obtain the Aconitin A injection for intrathecal injection with a specification of 0.5ml:0.04mg.
Embodiment 2
[0055] Embodiment 2. The prescription of Aconitin prefilled injection is (1000):
[0056] Aconitin 0.02g
[0057] Hydrobromic acid (47%) 0.006g
[0058] Water for injection 250ml
[0059] The preparation process of aconitin prefilled injection of the present invention is as follows: under 100-level laminar flow conditions, add 200ml of water for injection at 20-49°C in a beaker, carefully add 0.005g of hydrobromic acid (47%) under heat preservation Stir evenly, add 0.02 g of aconitin, continue to stir until dissolved, then add 0.006 g of hydrobromic acid (47%) dissolved in 10 ml of water for injection at 20 to 49 ° C under stirring to adjust the pH to 4.5 to 6.0, filter, and replenish Add water for injection at 20-49°C to a total volume of 250ml, filter with a 0.2μm membrane, and aseptically fill each 0.25ml into a pre-filled syringe to form a pre-filled 0.02mg of aconitin injection. According to the pharmacopoeia aconitin standard, it is tested by HPLC, and the related su...
Embodiment 3
[0060] Embodiment 3. Aconitin freeze-dried powder injection prescription is (1000):
[0061] Aconitine 0.20g
[0062] Hydrobromic acid 0.017ml (47%)
[0063] L-Mannitol 10g
[0064] The preparation process of the Aconitin freeze-dried powder injection of the present invention is as follows: under the condition of grade 100, the hydrobromic acid is first dissolved in 90ml of water for injection at 20-49°C to completely dissolve it; Add 0.2g of the element, dissolve under stirring, add water for injection at 20-49°C to 200ml, mannitol 10g, stir until dissolved, add water for injection at 20-49°C to 1000ml. Filtration, the filtrate is filtered through a 0.4 μm membrane, finely filtered by a 0.2 μm membrane, and the fine filtrate is sent to a packaging machine, divided into 1.0ml (2ml vials) per bottle, covered with a slotted lid, and sent to a freeze vacuum dryer. Quickly cool to -40°C for 2-3 hours, turn on the vacuum pump to reduce pressure, gradually increase the temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com