Preparation of nano-ferric fluoride and application of nano-ferric fluoride to positive electrode of high-specific-capacity lithium ion battery
An iron fluoride and nanotechnology, applied in battery electrodes, iron halide, nanotechnology, etc., can solve the problems of low conductivity, limited rate performance, etc., and achieve the effects of high yield, excellent electrochemical performance, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] (1) 0.1mol of Fe(NO 3 ) 3 9H 2 O was dissolved in 1L ethanol with a concentration of 1mol, and a colorless transparent solution was obtained under the action of ultrasound.
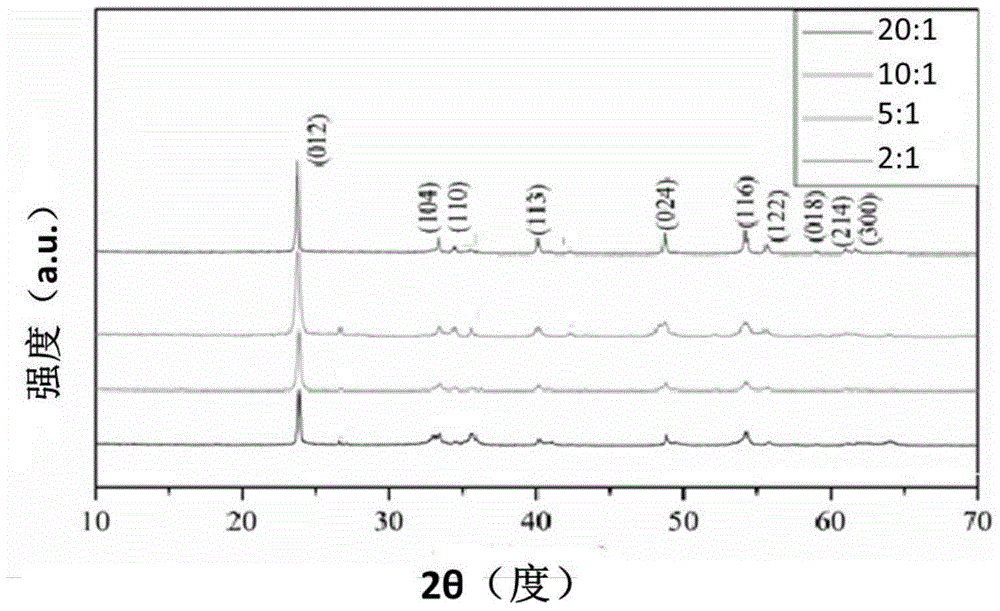
[0029] (2) 0.3mol of NH 4 HF 2 Dissolve in 1L deionized water, (NH 4 )HF 2 with Fe(NO 3 ) 3 9H 2 The mass ratio of O was 20:1.
[0030] (3) Pour the aqueous solution obtained in (2) into the ethanol solution obtained in (1), and stir vigorously for 1 min to obtain (NH 4 ) 3 FeF 6 White precipitate.
[0031] (4) The white precipitate in (3) was obtained by centrifugation, washed three times with ethanol, and dried in vacuum at 60°C for 12h to obtain (NH 4 ) 3 FeF 6 White powder.
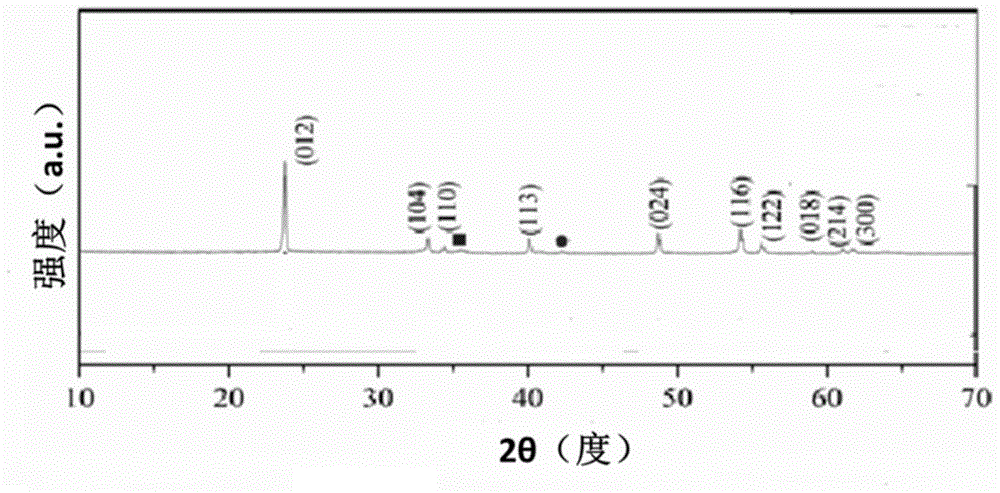
[0032] (5) (NH obtained in (4) 4 ) 3 FeF 6 The white powder is heated at 400°C for two hours under an argon atmosphere to obtain FeF 3 nanomaterials.
[0033] Above is FeF 3 Preparation of nanomaterials.
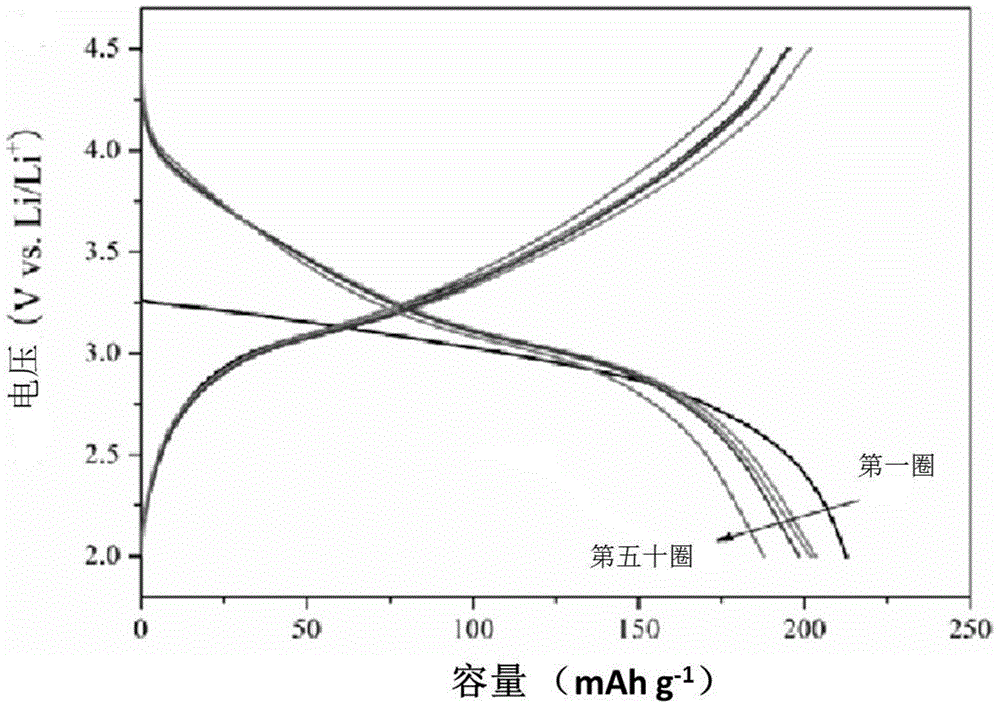
[0034] (6) FeF obtained in (5) 3 Nanomaterials, acetylene black, and polyvinylidene fluoride a...
example 2
[0045] (1) 0.1mol of Fe(NO 3 ) 3 9H 2 O was dissolved in 1L ethanol with a concentration of 2mol, and a colorless transparent solution was obtained under the action of ultrasound.
[0046] (2) 0.3mol of NH 4 HF 2 Dissolve in 1L deionized water, NH 4 HF2 with Fe(NO 3 ) 3 9H 2 The mass ratio of O was 10:1.
[0047] (3) Pour the aqueous solution obtained in (2) into the ethanol solution obtained in (1), and stir vigorously for 1 min to obtain (NH 4 ) 3 FeF 6 White precipitate.
[0048] (4) The white precipitate in (3) was obtained by centrifugation, washed three times with ethanol, and dried in vacuum at 60°C for 12h to obtain (NH 4 ) 3 FeF 6 White powder.
[0049] (5) (NH obtained in (4) 4 ) 3 FeF 6 The white powder is heated at 400°C for two hours under an argon atmosphere to obtain FeF 3 nanomaterials.
[0050] Above is FeF 3 Preparation of nanomaterials.
[0051] (6) FeF obtained in (5) 3 Nanomaterials, acetylene black, and polyvinylidene fluoride are m...
example 3
[0057] (1) 0.1mol of Fe(NO 3 ) 3 9H 2 O was dissolved in 1L ethanol with a concentration of 3mol, and a colorless transparent solution was obtained under the action of ultrasound.
[0058] (2) 0.3mol of NH 4 HF 2 Dissolve in 1L deionized water, NH 4 HF 2 with Fe(NO 3 ) 3 9H 2 The mass ratio of O is 5:1.
[0059] (3) Pour the aqueous solution obtained in (2) into the ethanol solution obtained in (1), and stir vigorously for 1 min to obtain (NH 4 ) 3 FeF 6 White precipitate.
[0060] (4) The white precipitate in (3) was obtained by centrifugation, washed three times with ethanol, and dried in vacuum at 60°C for 12h to obtain (NH 4 ) 3 FeF 6 White powder.
[0061] (5) (NH obtained in (4) 4 ) 3 FeF 6 The white powder is heated at 400°C for two hours under an argon atmosphere to obtain FeF 3 nanomaterials.
[0062] Above is FeF 3 Preparation of nanomaterials.
[0063] (6) FeF obtained in (5) 3 Nanomaterials, acetylene black, and polyvinylidene fluoride are m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com