Organic phosphaphenanthrene derivatives, and preparation method and application thereof
A technology of organophosphorus and derivatives, which is applied in the field of organophosphorus halogen-free flame retardants, can solve the problems of easy migration, poor flame retardant performance, high flame retardant dosage and high price, and achieve the expansion of application fields, improvement of flame retardant performance, Effect of improving flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1, preparation glycerol acrylate grafted DOPO
[0062] 1. Preparation of glycerol triacrylate
[0063] Get 25g of glycerol and 47.64 ml of acryloyl chloride, the molar ratio of the functional groups of hydroxyl and acid chloride is 1:1.2 at this time, mix glycerol and 300 ml of dichloromethane under the condition of ice-water bath, add 81.28 ml of Triethylamine was used as an acid-binding agent, and acryloyl chloride was added dropwise in an ice-water bath for half an hour, then warmed up to room temperature and stirred for 24 hours. The solution is light yellow, and there is a large amount of white insoluble powder at the same time.
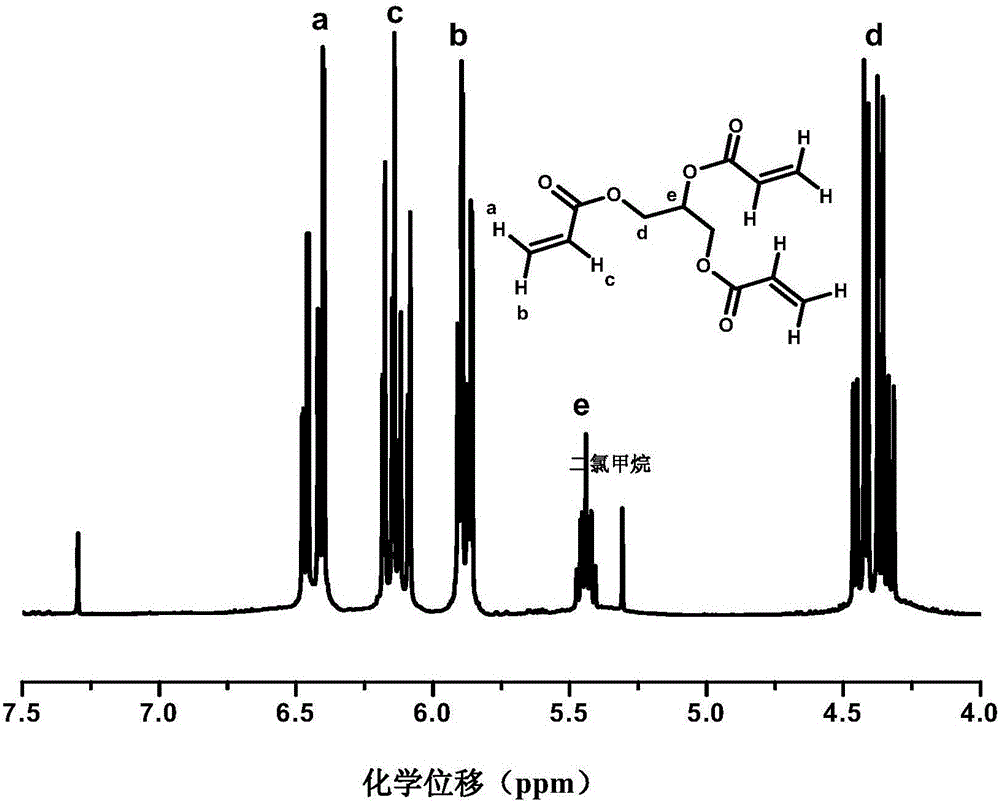
[0064] The product of the reaction was filtered, and the light yellow filtrate was taken and evaporated by rotary evaporation to remove excess acryloyl chloride and triethylamine. The product was dissolved with 250 ml of dichloromethane, extracted with 80 ml of saturated aqueous sodium bicarbonate solution each time, for a tot...
Embodiment 2
[0070] Embodiment 2, preparation glycerol acrylate graft DOPO
[0071] 1. Glycerol chemically grafted acrylic acid
[0072] Take 25 grams of glycerol and 67.07 milliliters of acrylic acid. At this time, the functional group ratio of hydroxyl and carboxyl groups is 1:1.2. Mix glycerol and 300 milliliters of dichloromethane at room temperature, and add 3.32 grams of 4-dimethyl Aminopyridine was used as a catalyst, 185 g of N,N-dicyclohexylcarbodiimide was added as a dehydrating agent, acrylic acid was added dropwise to the glycerin solution, and the reaction was stirred at room temperature for 24 hours. The solution is light yellow, and there is a large amount of white insoluble powder at the same time.
[0073] The reaction product was filtered, and the light yellow filtrate was taken, which was rotary evaporated to remove excess acrylic acid. The product is dissolved with 250 milliliters of dichloromethane, extracted three to four times with 80 milliliters of 5% hydrochloric...
Embodiment 3
[0077] Embodiment 3, preparation glycerol acrylate grafted DOPO
[0078] 1. Preparation of glycerol triacrylate
[0079] Using the same method as in Example 2, except that the temperature is raised to 80-120° C., the catalyst becomes 15.51 grams of monohydrate toluenesulfonic acid, and 1 gram of cuprous chloride is added as a polymerization inhibitor at the same time, and the propylene oxide is still obtained. Triol Triacrylate.
[0080] 2. Glycerol acrylate grafted DOPO
[0081] Using the same method as in Example 1, a glycerol triacrylate derivative grafted with DOPO was also obtained by proton nuclear magnetic resonance spectroscopy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com