The synthetic method of brominated epoxy resin
A technology of brominated epoxy resin and synthesis method, which is applied in the field of epoxy resin, and can solve problems such as high organic chlorine content, deep product color, and light product color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
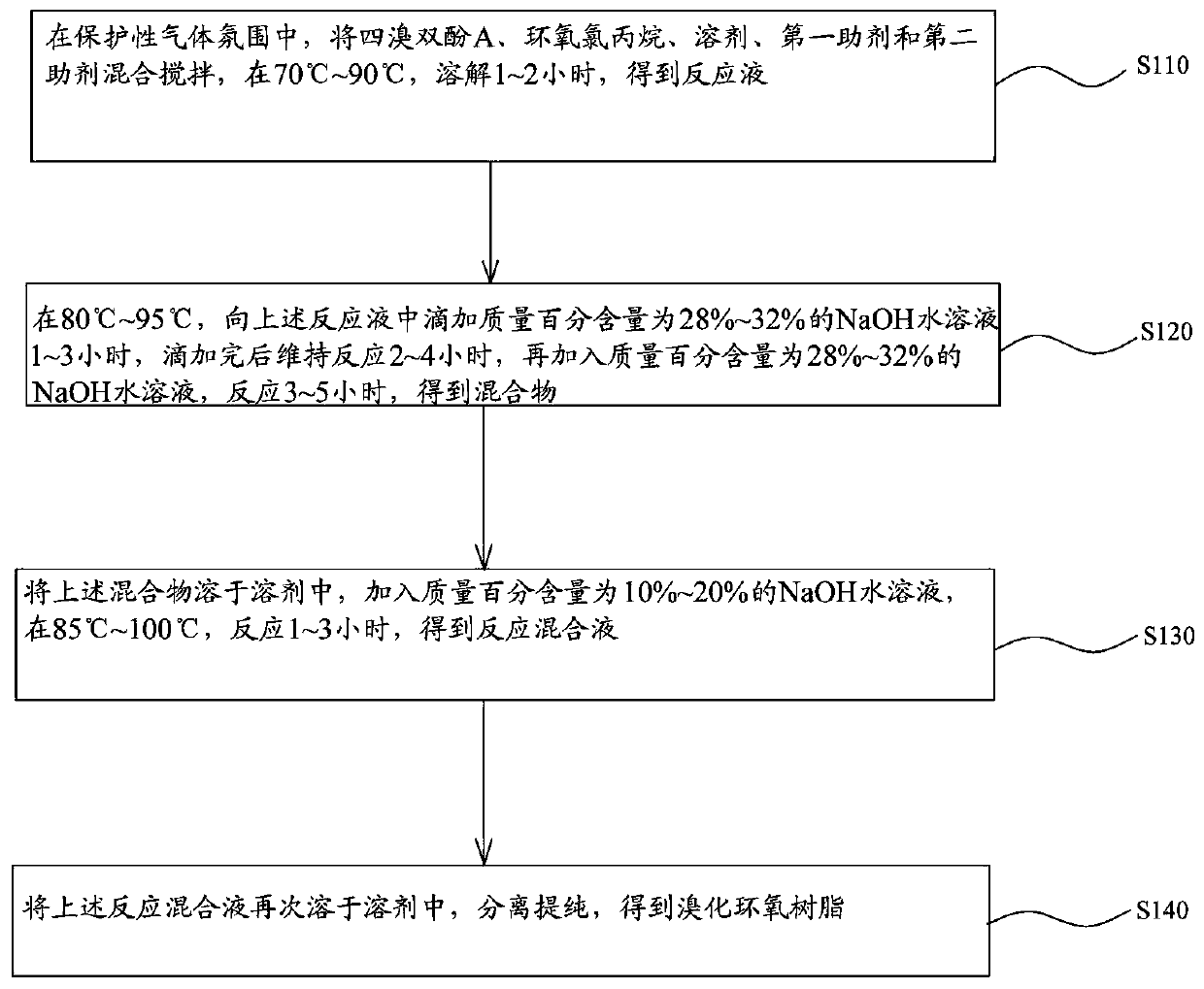
[0024] see figure 1 , the synthetic method of the brominated epoxy resin of an embodiment, comprises the following steps:
[0025] Step S110, in a protective gas atmosphere, mix and stir tetrabromobisphenol A, epichlorohydrin, solvent, the first auxiliary agent and the second auxiliary agent, and dissolve at 70°C~90°C for 1~2 hours to obtain The reaction solution.
[0026] Wherein, the protective gas is nitrogen.
[0027] The first auxiliary agent is selected from at least one of phosphite, pentaerythritol diphosphate, pentaerythritol diphosphite and thioester.
[0028] The second additive is selected from at least one of ethylene glycol, propylene glycol, n-butanol, isobutanol and neopentyl alcohol.
[0029] The solvent is at least one selected from toluene, benzene, xylene, methyl isobutyl ketone and n-butanol.
[0030] In this embodiment, in parts by mass, tetrabromobisphenol A is 272 parts, epichlorohydrin is 70-100 parts, solvent is 70-140 parts, the first auxiliary a...
Embodiment 1
[0046] In a nitrogen atmosphere, 272g of tetrabromobisphenol A, 70g of epichlorohydrin, 70g of methyl isobutyl ketone, 1g of phospholipid and 5g of ethylene glycol were mixed and stirred, and dissolved at 70°C for 1 hour to obtain a reaction solution. At 80° C., 10 g of 30% NaOH aqueous solution was added dropwise to the above reaction solution for 1 hour, then the reaction was maintained for 2 hours, 150 g of 30% NaOH aqueous solution was added, and the mixture was obtained for 3 hours. The above mixture was dissolved in 300 g of methyl isobutyl ketone, 10 g of 10% NaOH aqueous solution was added, and the mixture was reacted at 85° C. for 1 hour to obtain a reaction mixture. Dissolve the above reaction mixture in methyl isobutyl ketone again, add 10g of phosphoric acid and 80g of water, wash with water several times until neutral, separate the liquids, take the organic layer, and distill under reduced pressure to separate the solvent to obtain brominated epoxy resin .
[004...
Embodiment 2
[0059] In a nitrogen atmosphere, 272g of tetrabromobisphenol A, 100g of epichlorohydrin, 140g of toluene, 10g of pentaerythritol diphosphate and 15g of propylene glycol were mixed and stirred, and dissolved at 90° C. for 2 hours to obtain a reaction solution. At 95° C., 25 g of 28% NaOH aqueous solution was added dropwise to the above reaction solution for 3 hours, and the reaction was maintained for 4 hours. 250 g of 28% NaOH aqueous solution was added and reacted for 5 hours to obtain a mixture. The above mixture was dissolved in 500 g of toluene, 30 g of 20% NaOH aqueous solution was added, and the mixture was reacted at 100° C. for 3 hours to obtain a reaction mixture. The above reaction mixture was dissolved in toluene again, 110 g of water was added, washed with water several times until neutral, separated, the organic layer was taken, and the solvent was separated by distillation under reduced pressure to obtain a brominated epoxy resin.
[0060] After measurement, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar mass | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com