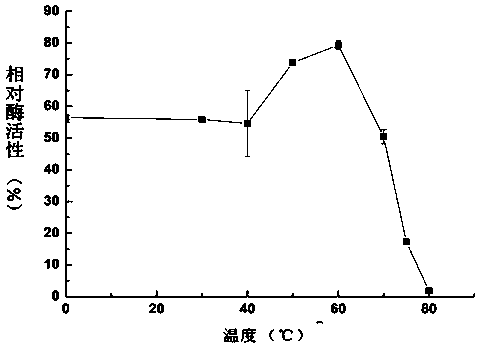
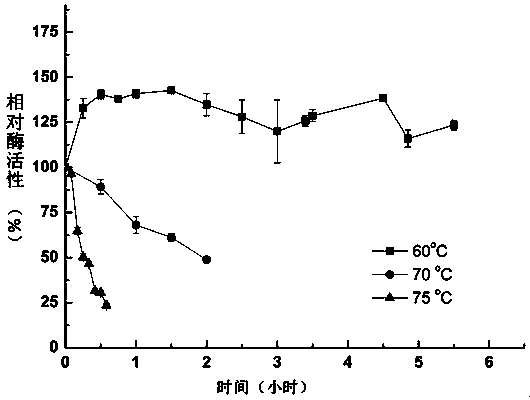
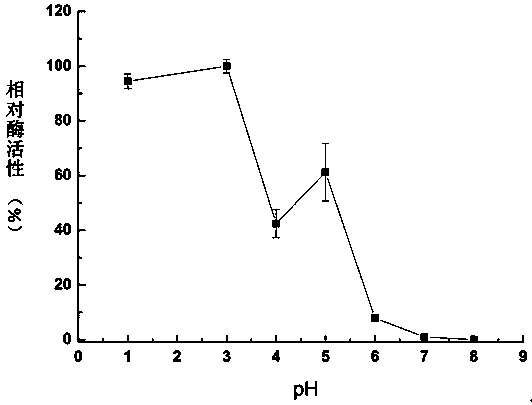
A kind of thermostable Trametes trichos laccase ttlac13 and its application
A thermostability, technology of Trametes trichotis, applied in the field of enzyme engineering and genetic engineering, can solve the problem of low thermal stability of laccase strain Trametes trichosum, achieve good industrial application prospects, good economic benefits and social effects, improve efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Laccase produced by Trametes trichotillosum and the purification of laccase TtLac13
[0034] The laccase strains used were screened by our laboratory and identified as T. trogii For typical strains, put this strain in a medium containing glucose 20 g / L, yeast extract 5 g / L, peptone 5 g / L, MgSO 4 .7H 2 O 1 g / L, plus 15 g / L agar on a solid medium plate or a large test tube for bacterial strain activation. After cultivating for 3 days, break the activated bacterial strain with sterilized glass beads, and then take the broken The final bacterial solution was placed in a solution containing 28 g / L of glucose, 10 g / L of yeast extract, 10 g / L of peptone, FeSO 4 0.4 mM, CuSO4 1.65 mM, Tween-80 6 g / L, PEG 4000 45 mg / L in the optimized enzyme-producing liquid medium, make three groups and place them in a shaker at 28°C at 180rpm.
[0035]Collect the above cultured culture medium, centrifuge at 5000 rpm, 4 °C for 5 min to collect the supernatant, which is the cru...
Embodiment 2
[0036] Example 2: Determination of protein concentration
[0037] The protein concentration at each stage of the laccase purification process was determined by the Coomassie Brilliant Blue method. Take 0.1 mL of properly diluted purified laccase and add it to 5 mL of Coomassie Brilliant Blue G-250 reagent, that is, add 95% ethanol to a mortar with 100 mg of Coomassie Brilliant Blue G-250 and grind to dissolve it, and take the upper layer The liquid was placed in a beaker, and 10 mL of 95% ethanol was added and ground in a mortar, repeated 3 times to dissolve the Coomassie brilliant blue, and the mortar was washed with 20 mL of ethanol several times. Add 100 mL of 85% concentrated phosphoric acid to the beaker in portions, and then dilute to 1 L with deionized water. After reacting for 2-5 minutes, measure the light absorption value of the reaction solution at 595 nm on a spectrophotometer. To draw a standard curve, add 1 mg / L standard protein solution containing 100 mg / L bov...
Embodiment 3
[0038] Embodiment 3: enzyme activity assay method
[0039] Using ABTS as the substrate, the increase in the absorbance value of the reaction solution at 420 nm was measured within 3 min. First prepare 100 mM phosphate-citrate buffer, pH 4.0. And use this buffer to prepare 2 mM ABTS solution. Use 1.5 mL reaction system: 1.1 mL phosphate-citrate buffer (100 mM, pH 4.0) containing 2 mM ABTS and 0.4 mL laccase pure enzyme diluted with phosphate-citrate buffer (100 mM, pH 4.0) liquid. Record the change of the absorbance value of the reaction solution at 420 nm within 3 min of reaction. Define the amount of enzyme needed to oxidize 1 μmol ABTS in one minute as 1 enzyme activity unit (U). The pure enzyme solution of laccase after heat inactivation was used as the control, and all experiments were repeated in triplicate.
[0040] Laccase activity calculation formula U(U / L) = N×V 1 ×(A 3 -A 0 ) / (ε×L) / V 2 / t
[0041] N is the dilution factor, V 1 is the total reaction volume,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com