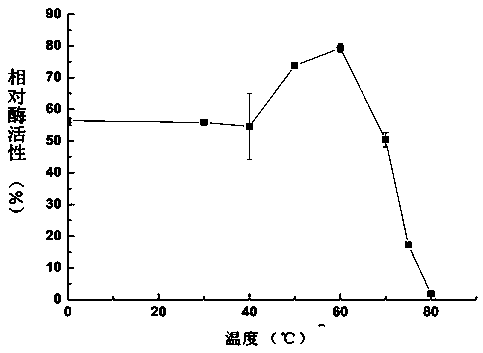
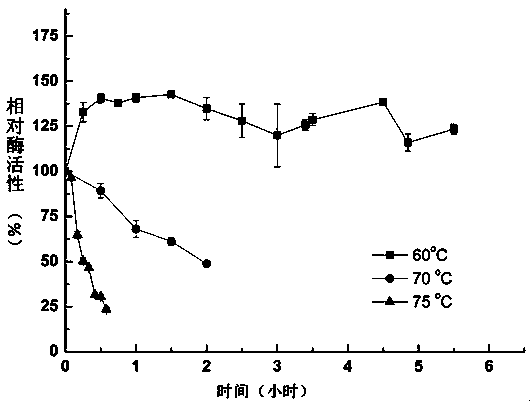
Heat-stability trametes trogii laccase TtLac13 and application thereof
A technology for thermal stability and Trametes pilaris, which is applied in the fields of enzyme engineering and genetic engineering, can solve the problem of low thermal stability of laccase of Trametes pilus strains, achieves good industrial application prospects, good economic benefits and social effects, and improves the The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Purification of laccase produced by Trametes trichotillosum and laccase TtLac13
[0034] The laccase strain used was screened by our laboratory and identified as a typical strain of T. trogii. This strain was placed in a place containing 20 g / L of glucose, 5 g / L of yeast extract, 5 g / L of peptone, MgSO 4 .7H 2 O1g / L, add 15g / L agar on the solid medium plate or large test tube to activate the strain, after culturing for 3 days, crush the activated strain with sterilized glass beads, and then take the crushed bacterial liquid Put in glucose 28g / L, yeast extract 10g / L, peptone 10g / L, FeSO 4 0.4mM, CuSO4 1.65mM, Tween-806g / L, PEG400045mg / L optimized enzyme-producing liquid medium, make three groups and place them in a shaker at 28°C at 180rpm.
[0035]Collect the above cultured culture medium, centrifuge at 5000rpm at 4°C for 5min to collect the supernatant as the crude enzyme liquid; first use ammonium sulfate with a saturation of 80% to precipitate the pro...
Embodiment 2
[0036] Example 2: Determination of protein concentration
[0037] The protein concentration at each stage of the laccase purification process was determined by the Coomassie Brilliant Blue method. Take 0.1 mL of properly diluted purified laccase and add it to 5 mL of Coomassie Brilliant Blue G-250 reagent, that is, add 95% ethanol to a mortar with 100 mg of Coomassie Brilliant Blue G-250 to dissolve it, and take the upper liquid Put it in a beaker, add 10mL of 95% ethanol and grind it in a mortar, repeat 3 times to dissolve Coomassie Brilliant Blue, and wash the mortar with 20mL of ethanol several times. Add 100mL of 85% concentrated phosphoric acid to the beaker in portions, and then dilute to 1L with deionized water. After reacting for 2-5 minutes, measure the light absorption value of the reaction solution at 595 nm on a spectrophotometer. To draw a standard curve, add 1 mg / L standard protein solution containing 100 mg / L bovine serum albumin to each test tube: 0, 0.01, 0....
Embodiment 3
[0038] Embodiment 3: enzyme activity assay method
[0039] Using ABTS as the substrate, measure the increase in the absorbance value of the reaction solution at 420 nm within 3 minutes. First prepare 100 mM phosphate-citrate buffer, pH 4.0. And use this buffer to prepare 2mM ABTS solution. Use 1.5mL reaction system: 1.1mL phosphate-citric acid buffer (100mM, pH 4.0) containing 2mMABTS and 0.4mL laccase pure enzyme solution diluted with phosphate-citric acid buffer (100mM, pH 4.0). Record the change in the absorbance value of the reaction solution at 420 nm within 3 minutes of reaction. Define the amount of enzyme needed to oxidize 1 μmol ABTS in one minute as 1 enzyme activity unit (U). The pure enzyme solution of laccase after heat inactivation was used as the control, and all experiments were repeated in triplicate.
[0040] Laccase activity calculation formula U(U / L)=N×V 1 ×(A 3 -A 0 ) / (ε×L) / V 2 / t
[0041] N is the dilution factor, V 1 is the total reaction volum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com