Monoclonal antibody resisting ovarian cancer and application thereof
A monoclonal antibody and ovarian cancer technology, applied in the field of medical diagnosis, can solve the problems of patients losing radical surgery, atypical signs, and low specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
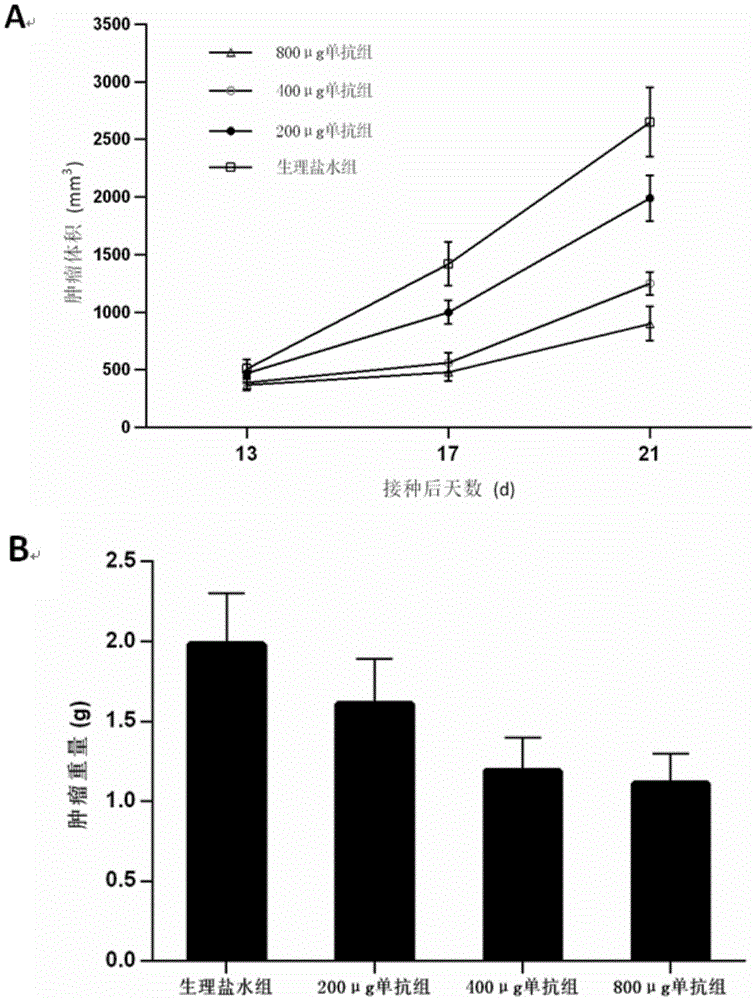
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 hybridoma
[0024] 1.1 Animal immunization Take 6-8 weeks old female BALB / c mice, each with 1-2×10 ovarian cancer cells 6 SK-OV-3 cells were immunized by intraperitoneal injection 4 times at intervals of 3 weeks. Blood was collected from the inner canthus of the mice before each immunization, and the antibody titer of the mouse serum was detected by indirect cell ELISA. When the serum antibody titer of the immunized mice reached the maximum and no longer increased, the mouse splenocytes were taken for fusion. 3 days before fusion Boost immunity 1 time.
[0025] 1.2 Indirect cell ELISA test Inoculate 2×10 cells on a 96-well plate 5 SK-OV-3 cells per well (Shanghai Baili Biotechnology Co., Ltd.), until the cell growth and confluence reached 80%, fixed with 95% ethanol, washed 3 times with PBS, permeabilized with 0.2% Triton-X-100 for 20 min, and then used 50g / LBSA was blocked at 37°C for 2 hours, 100 μL of immunized mouse serum of differ...
Embodiment 2
[0027] Example 2 Preparation and purification of monoclonal antibody ascites
[0028] Female BALB / c mice aged 8-10 weeks were injected intraperitoneally with 0.5 mL of paraffin oil, and 10 days later, they were injected intraperitoneally with well-growing hybridoma cells NM003-1 and NM003-2 at approximately 1×10 6 After 1-2 weeks, the ascites was aspirated, after 1 hour at 37°C, and overnight at 4°C, the ascites were centrifuged the next day and purified by ProteinG affinity chromatography to obtain purified monoclonal antibodies NJ003-1 and NJ003-2 .
Embodiment 3
[0029] Identification of embodiment 3 monoclonal antibody
[0030] 3.1 Identification of monoclonal antibody Ig subclass: the purified monoclonal antibody was diluted with PBS 1:10000, and operated according to the instructions of the assay kit. The subclasses of monoclonal antibodies NJ003-1 and NJ003-2 are IgG, and the light chain is κ chain.
[0031] 3.2 Determination of monoclonal antibody titer: Dilute the purified monoclonal antibodies NJ003-1 and NJ003-2 with PBS, respectively, take 100 μL and add them to 96-well plates coated with SK-OV-3 cells, and use indirect cell ELISA A 450 The titer is the maximum dilution of the monoclonal antibody that can immunoreact with the coated cells. The titer of monoclonal antibody NJ003-1 is 8×10 5 , the titer of monoclonal antibody NJ003-2 was 4×10 5 . The hybridoma cell line NM003-1 was deposited in China Center for Type Culture Collection on August 31, 2011, the deposit address is Wuhan, Wuhan University, and the deposit number...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com