One-step synthesis method for novel water-soluble 8-hydroxy porphyrin
A synthesis method and water-soluble technology are applied in the field of one-step synthesis of novel water-soluble porphyrins, which can solve problems such as troublesome post-processing, and achieve the effects of simple post-processing, good water solubility, biocompatibility, and high reaction yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
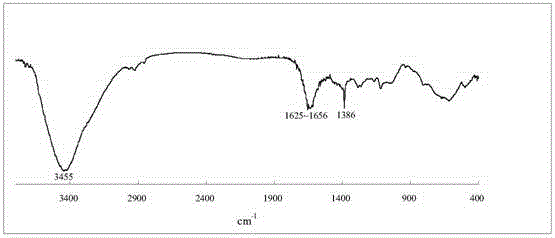
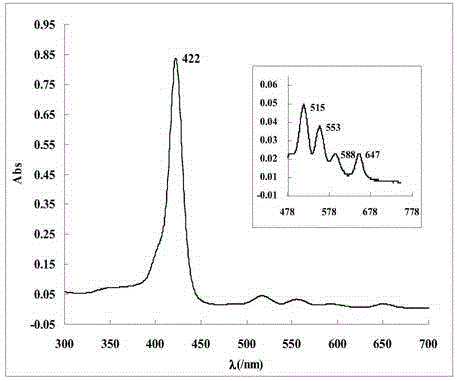
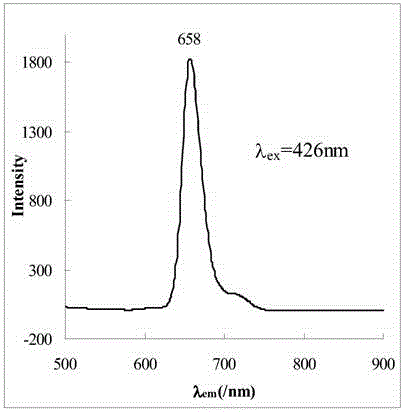
[0039] The name of the water-soluble porphyrin compound is 5,10,15,20-tetra-(3,5-dihydroxyphenyl)porphyrin, and the specific synthesis steps and characteristics are as follows:
[0040] figure 1 It is the synthetic route of this compound, using 40mL propionic acid / acetic acid (3:2; V / V) as the reaction solvent, heating to reflux to 120℃, and putting 1.4 g of 3,5-dihydroxybenzaldehyde into the mixed acid under vigorous stirring Dissolve in the medium, about 0.68mL (0.68g) of freshly steamed pyrrole is added dropwise within 2min, reflux is continued for 45min, and then the mixed acid is removed by distillation under reduced pressure. Dissolve the crude product with 30 mL methanol, add 10 mL silica gel (100-150 mesh) to adsorb the product, and distill off the methanol solvent. The adsorbed product is separated by column chromatography with the same silica gel, and the sample is loaded by dry method. 2 Cl 2 And CH 2 Cl 2 / Methanol mixed solvent (10:1; V / V) was used as eluent for grad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com