A kind of preparation method of battery cathode material lithium iron phosphate
A lithium iron phosphate, battery positive electrode technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of high price, cycle performance, poor thermal stability and high temperature performance, and toxic cobalt, so as to reduce the formation of gaps The probability of improving the electrochemical performance and the effect of increasing the tap density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
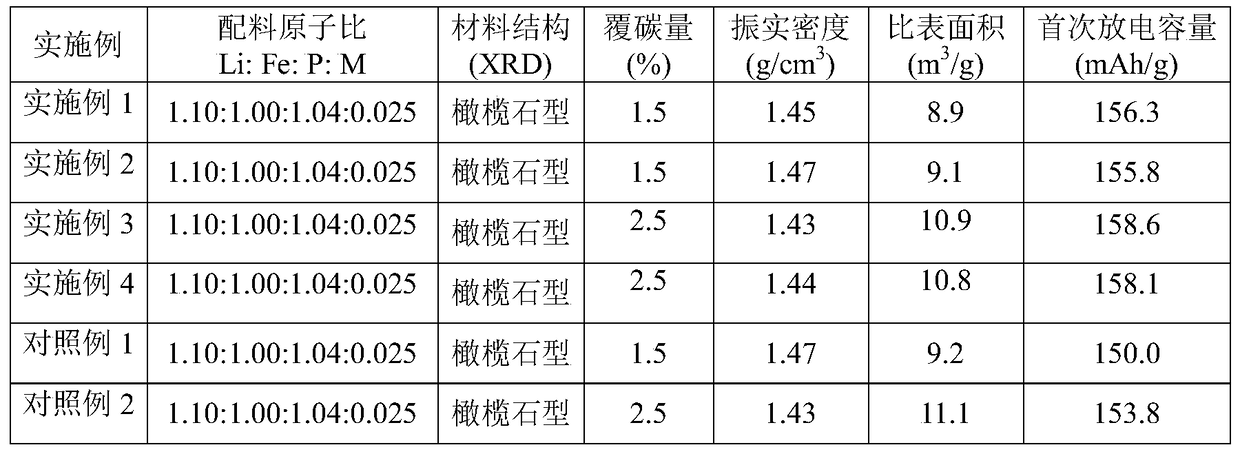
Embodiment 1
[0023] (1) Primary carbon coating: according to the molar ratio of Li:Fe:P:M=1.10:1.00:1.04:0.025, weigh 0.5468kg lithium hydroxide (LiOH·H 2 O, purity 99.5%), 0.0513kg lithium dihydrogen phosphate (LiH 2 PO 4 , purity 99.3%), 2.3354kg iron phosphate (FePO 4 2H 2 O, purity 98.00%), 0.0684kg dopant M (Al(NO 3 ) 3 9H 2 O, Mg(NO 3 ) 2 , Mn(NO 3 ) 2 , Co(NO 3 ) 2 ·6H 2 0), add 3000ml of water, and adopt ball milling for 8h to obtain a mixed slurry, the D50 of which is less than 1 μm; transfer the ball milled slurry to a reaction kettle, heat up to 100°C while stirring, and stir at a constant temperature for 10h; mix 0.1440kg of sucrose (C 12 h 22 o 11 ) was dissolved in 150ml of water, the sucrose aqueous solution was added to the reaction kettle, and the constant temperature stirring was continued for 1 hour; the formed stirred slurry was spray-dried to obtain a dry material, and the drying temperature was 80-200°C;
[0024] (2) Secondary carbon coating: Under the...
Embodiment 2
[0027] (1) Primary carbon coating: according to the molar ratio of Li:Fe:P:M=1.10:1.00:1.04:0.025, weigh 0.5468kg lithium hydroxide (LiOH·H 2 O, purity 99.5%), 0.0513kg lithium dihydrogen phosphate (LiH 2 PO 4 , purity 99.3%), 2.3354kg iron phosphate (FePO 4 2H 2 O, purity 98.00%), 0.0684kg dopant M (Al(NO 3 ) 3 9H 2 O, Mg(NO 3 ) 2 , Mn(NO 3 ) 2 , Co(NO 3 ) 2 ·6H 2(0), add 3000ml of water, and adopt ball milling for 8h to obtain a mixed slurry, the D50 of the mixed slurry is less than 1 μm; transfer the ball milled slurry to a reaction kettle, heat up to 200°C while stirring, and stir at a constant temperature for 2.5h; 0.1440kg Sucrose (C 12 h 22 o 11 ) was dissolved in 150ml of water, the sucrose aqueous solution was added to the reaction kettle, and the constant temperature stirring was continued for 1 hour; the formed stirred slurry was spray-dried to obtain a dry material, and the drying temperature was 80-200°C;
[0028] (2) Secondary carbon coating: Unde...
Embodiment 3
[0031] (1) Primary carbon coating: according to the molar ratio of Li:Fe:P:M=1.10:1.00:1.04:0.025, weigh 0.5468kg lithium hydroxide (LiOH·H 2 O, purity 99.5%), 0.0513kg lithium dihydrogen phosphate (LiH 2 PO 4 , purity 99.3%), 2.3354kg iron phosphate (FePO 4 2H 2 O, purity 98.00%), 0.0684kg dopant M (Al(NO 3 ) 3 9H 2 O, Mg(NO 3 ) 2 , Mn(NO 3 ) 2 , Co(NO 3 ) 2 ·6H 2 0), add 3000ml of water, and adopt ball milling for 8h to obtain a mixed slurry, the D50 of which is less than 1 μm; transfer the ball milled slurry to a reaction kettle, heat up to 100°C while stirring, and stir at a constant temperature for 10h; mix 0.1440kg of sucrose (C 12 h 22 o 11 ) was dissolved in 150ml of water, and the sucrose aqueous solution was added to the reaction kettle, and continued to stir at a constant temperature for 2 hours; the formed stirred slurry was spray-dried to obtain a dry material, and the drying temperature was 80-200°C;
[0032] (2) Secondary carbon coating: Under th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com